BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijt.arakmu.ac.ir/article-1-1451-en.html


 , Afni Mayzida1
, Afni Mayzida1 

 , Aninditha Dewi *2
, Aninditha Dewi *2 

 , Nafisa Kaffah1
, Nafisa Kaffah1 

 , Nurfadhiya Maharani1
, Nurfadhiya Maharani1 
 , Fransiska Pontjosudargo3
, Fransiska Pontjosudargo3 
 , Evy Shavilla4
, Evy Shavilla4 
 , Aprilia Sweetasari5
, Aprilia Sweetasari5 

2- Pharmacology, Department of Pharmacology, School of Medicine, Jenderal Achmad Yani University, Cimahi, Indonesia ,
3- Anatomy, Department of Anatomy, School of Medicine, Jenderal Achmad Yani University, Cimahi, Indonesia
4- Ear, Nose, and Throat Specialist, Department of Ear, Nose, and Throat, School of Medicine, Jenderal Achmad Yani University, Cimahi, Indonesia
5- Neurologist, Department of Neurologi, School of Medicine, Jenderal Achmad Yani University, Cimahi, Indonesia
Introduction
Avocado is one of the most popular fruits in various countries, including Indonesia, due to its many health benefits [1]. So far, the utilization of avocados has only involved consuming the flesh, resulting in the waste of avocado peels and contributing to environmental issues related to increased waste disposal. Avocado peels can be utilized as herbal tea and traditional medicine. The culture of drinking tea and herbal medicine has become ingrained among the Indonesian people [2,3]. Avocado is known as a plant that has a high content of nutrients and phytochemicals. The phytochemicals found in avocados include phenolic compounds such as flavonoids, phenolic acids, tannins, and saponins, as well as other compounds such as phytosterols, alkaloids, and carotenoids. The presence of these compounds makes the utilization of avocados not limited to the pulp, but also includes other parts, such as leaves, seeds, and peels, which have a higher phenolic content compared to the pulp. Avocado peel has potential for utilization in the medical field.
The abundance of phytochemical compounds in avocado peel is an effective anti-inflammatory, antibacterial, and antifungal agent [4,5]. Based on research, as an anti- inflammatory, the compounds in avocado peel can neutralize free radicals and deactivate other prooxidants, while also exhibiting anti-inflammatory effects by obstructing the activation of key cellular signalling pathways that are responsible for initiating systemic inflammation.6 Another benefit, as an antimicrobial, is its effectiveness against Escherichia coli, Staphylococcus aureus, and Staphylococcus epidermidis [6–9]. In addition, avocado peel extract shows very good antifungal effects against the fungus Trichophyton rubrum, the fungus responsible for causing dermatophytosis [9].
Despite the numerous health benefits of herbal medicine, it is crucial to understand its safety to prevent any potential harmful effects. Toxicity testing is a test aimed at identifying the toxic effects of a substance on biological systems and collecting characteristic dose- response data from the test preparation.
The information obtained from this test can be used to determine the level of danger of the test substance when exposed to humans, as well as to determine the appropriate dosage to be used [10]. The purpose of conducting acute toxicity tests is to identify any toxic effects that may occur shortly after the oral administration of the test preparation. Based on the Organization of Economic Co-operation and Development (OECD), there are three methods in acute toxicity testing, namely Fixed Dose, Acute Toxic Class, and Up and Down Procedure. This research employs the Acute Toxic Class Method, which prioritizes animal welfare by utilizing only three animals at each stage [10].
Materials and Methods
The avocado peels used in this research come from PT Spektani Berkah Argo's avocado plantation in Bogor, West Java. The tools used in this research are a sonde pipette, rotary evaporator, special weighing device for laboratory animals, extract container, analytical balance, and CO2 for euthanasia.
The subjects of this research were female ddY strain mice (Mus musculus). Female mice are slightly more sensitive compared to male mice, due to hormonal factors [11]. In this research, the mice were obtained from the Biofarma Laboratory in Bandung, weighing 30 grams, and were not pregnant.
The avocado peels were thinly sliced, totalling 1 kg, and dried at a temperature of 60°C. Then, the dried peels were ground using a grinder until they became a simplicia powder, resulting 235 grams. Then, the simplicia of avocado peels is soaked in 96% ethanol at a ratio of 1:10 for 3x24 h while being stirred occasionally. Maceration is stored in a place protected from sunlight. Next, filtration was carried out using filter paper to obtain the liquid from the soaking process, which was then evaporated using a rotary evaporator, resulting in 59 grams of avocado peel extract.
In this research, the Acute Toxic Class method was used (Figure 1), the principle of this testing method is the gradual administration of the test preparation to test animals with the minimum number of animals. The initial doses used usually consist of four fixed dose levels, namely 5, 50, 300, and 2,000 mg/kg body weight. The administration of the test preparation in stages uses three test animals, which are generally female, at each stage.[10] The presence or absence of test animals experiencing death determines the next testing phase. This research divided the animals into five groups consisting of control group (CG), 300 mg/kgBW single dose group (G1), 300 mg/kgBW repeated dose group (G2), 2,000 mg/ kgBW single dose group (G3), and 2,000 mg/ kgBW repeated dose group (G4). The initial dose given was 300 mg/KgBW because based on research, the effective dose of avocado peel ethanol extract is 150 mg/KgBW [12]. The test preparation was then administered orally using a feeding tube to the test group, and standard feed and water were provided to the control group. Afterwards, observations were made during the first 30 min, then the first 4 h, and throughout the 24-h period. If there are two or three animals that die in the group receiving the test preparation, testing is conducted by lowering the dose until there is only one death or no test animals die. Meanwhile, if no test animals die or only one animal dies, testing is conducted with the same dose or an increase to the highest dose (2,000 mg/KgBW) in a different group of animals [10].
Determination of total phenols, flavonoids, tannins, saponins, and alkaloids
The peel of avocado fruit has the potential to act as an antibacterial, antioxidant, antifungal, and anti- inflammatory due to its content of alkaloids, saponins, tannins, phenols, and flavonoids. According to previous studies, the phytochemicals contained in 100 grams of avocado peel extract found phenolic compounds amounting to 21.833 ± 0.118 mg; total flavonoids amounting to 2.607 ± 0.111 mg; tannins amounting to
38.357 ± 0.202; saponins amounting to 8.874% ±
0.031%; and total alkaloids amounting to 9.95 ± 0.035 mg [13]. Compared to the concentration of flavonoid compounds in avocado leaves (1.5289%), avocado peels are higher (2.4313%), and in avocado seed extracts, the flavonoid content is 2.14 mg/100g, while in avocado flesh, the total phenolic compound content is 12.800 mg/100g [5,14,15].
The death of mice was calculated in the first 24 h since the administration of ethanol extract of avocado peel by observing every 4 h. Furthermore, the observation of mice deaths is carried out once a day until day 14. Observations are conducted by looking for signs of death, such as the absence of a pulse, absence of respiratory movements (observed by examining the movement of the chest wall), absence of corneal reflexes, no responses to pinching, grayish mucosa and rigor mortis. Rigor mortis is the definitive sign of death among all the previously mentioned signs. Animals that are dying or showing severe toxic symptoms must be euthanized. The final step is to record the number of animals that died during the test, the animals that were sacrificed, and the time of death for each animal.
This research measures the weight of mice from the beginning to the end of the experiment. After the administration of the avocado peel extract, the mice were observed for 24 h. If there were no deaths, the body weight of the mice was measured from day 1 to day 14 to assess any changes in their body weights. The measurement was conducted using a digital scale by preparing and calibrating the device to zero, then weighing the mice one by one. Changes in body weight before and after administration of the avocado peel extract were assessed within each group and compared between the control and test group.
Observation of toxic symptoms shown as behavioral changes in mice
Observation of toxicity symptoms was made by examining the behavioral changes, such as motor activity, Straub phenomenon, piloerection, ptosis, pineal reflex, corneal reflex, lacrimation, posture, hanging, reestablishment, flexion, Haffner, tremor, seizures, salivation, and defecation [16].The observed toxicity effects include pharmacological observations of the neuromuscular system, central nervous system, sensory nervous system, autonomic nervous system, digestive system, and eye and peel organs of mice [17,18]. Observations were made at 30, 60, 120, and 240 min after administration of the extract, then every 4 h for the first 24 h, and once a day until day 14th [16].
Macroscopic changes in liver and kidney
The parameters observed in this research are the color, size, and relative weight of the liver and kidney organs of the mice. The color of the liver and kidneys was analyzed visually to determine whether there was a change in organ color between the experimental group and the control group. Observation of the size of the liver and kidney was carried out by comparing the length, width, and thickness of the organs in the experimental group with the control group to see if there was a change in size after the treatment. The relative weight of the organs were measured using the formula:
Relative organ weight (%)
Organ weight (grams)
![]() = x 100%
= x 100%
Body weight of test animals (gram)
Results
Effect of Avocado Peel Extract on the Number of Deaths
The results of the first 24 h of initial observation showed no deaths in mice in all treatment groups, namely G1, G2, G3, G4, and CG. Further monitoring continued until day 14 to confirm the absence of deaths within that time span. The results indicated that by day 14, no deaths had occurred in any of the treatment groups. Therefore, the total number of deaths recorded during this study period was zero in all groups, regardless of the low or high doses administered.
Effect of Avocado Peel Extract on Body Weight of Mice
Body weight is the most visible indicator of toxic effects after a toxic substance is administered. Significant weight loss is an indicator of toxic effects or a severe health condition. The average body weight before and after the research were obtained as follows.
Table 1 shows a decrease in the body weight of mice in each treatment group compared to the control group, with the results of the Kruskal Wallis test analysis indicating no significant change in body weight as the P-value obtained was 0.096 (P>0.05) between the control group and the treatment group. This indicates that the weight loss occurring in each treatment group is almost the same, without any significant differences.
Table 1. Effect of avocado peel extract on the body weight of female mice before and after treatment with statistical test results
|
Group |
|
Mean (gram) ± SD |
|
P-value |
|
Pre-treatment body weight |
Post-treatment body weight |
Body weight changes |
||
|
CG |
30.67 ± 1.15 |
32.00 ± 2.22 |
1.33 ± 1.19 |
|
|
G1 |
32.00 ± 1.73 |
28.69 ± 1.22 |
-3.31 ± 2.18 |
|
|
G2 |
36.67 ± 3.05 |
34.71 ± 2.48 |
-1.95 ± 1.03 |
0.096 |
|
G3 |
33.67 ± 2.30 |
30.81 ± 1.59 |
-2.86 ± 0.79 |
|
|
G4 |
34.67 ± 6.11 |
32.57 ± 6.00 |
-2.09 ± 1.39 |
|
|
Note: Control Group (CG), 300 mg/KgBW single dose (G1), 300 mg/KgBW repeat dose (G2), 2000 mg/KgBW single dose (G3), and 2000 mg/KgBW repeat dose (G4) |
||||
Effect of Avocado Peel Extract on Behavioral Changes in Mice
Observation of toxicity symptoms, which was conducted by observing behavioral changes at 30, 60, 120, and 240 min after the administration of the extract, is shown in Table 2. Table 2 shows the observed data on the presence of toxic symptoms to determine the organs and systems affected by the extract. The presence or absence of tremor indicates toxic symptoms of the central nervous system; toxic symptoms of the sensory nervous system are observed
through the pineal reflex, corneal reflex, flexion, and Haffner; and toxic symptoms of the autonomic nervous system are observed through salivation and lacrimation. Motor activity and the Straub phenomenon were performed to observe toxic symptoms of the neuromuscular system, and defecation was done to observe toxic symptoms of the digestive system. Ptosis is an eye toxicity symptom, and piloerection is a toxic symptom affecting the skin [19,20].
Table 2. Effect of avocado peel extract on behavioral changes of female mice after the treatment
|
Symptoms observed |
Percentage of appearance in mice (%) |
|||||
|
|
CG |
G1 |
G2 |
G3 |
G4 |
|
|
|
Normal |
100 |
100 |
100 |
100 |
100 |
|
Motoric Activity |
Increase |
0 |
0 |
0 |
0 |
0 |
|
|
Decrease |
0 |
0 |
0 |
0 |
0 |
|
Straub Phenomenon |
|
0 |
0 |
0 |
0 |
0 |
|
Piloerection |
|
0 |
0 |
0 |
0 |
0 |
|
Ptosis |
|
0 |
0 |
0 |
0 |
0 |
|
Pineal Reflex |
|
100 |
100 |
100 |
100 |
100 |
|
Corneal Reflex |
|
100 |
100 |
100 |
100 |
100 |
|
Lacrimation |
|
0 |
0 |
0 |
0 |
0 |
|
Posture |
Normal |
100 |
100 |
100 |
100 |
100 |
|
Abnormal |
0 |
0 |
0 |
0 |
0 |
|
|
Hanging |
|
100 |
100 |
100 |
100 |
100 |
|
Reestablishment |
|
100 |
100 |
100 |
100 |
100 |
|
Flexion |
|
100 |
100 |
100 |
100 |
100 |
|
Haffner |
|
100 |
100 |
100 |
100 |
100 |
|
Tremor |
|
0 |
0 |
0 |
0 |
0 |
|
Seizures |
|
0 |
0 |
0 |
0 |
0 |
|
Salivation |
|
0 |
0 |
0 |
0 |
0 |
|
Defecation |
Normal |
100 |
100 |
100 |
100 |
100 |
|
Abnormal |
0 |
0 |
0 |
0 |
0 |
|
Effect of Avocado Peel Extract on Macroscopic Liver and Kidneys
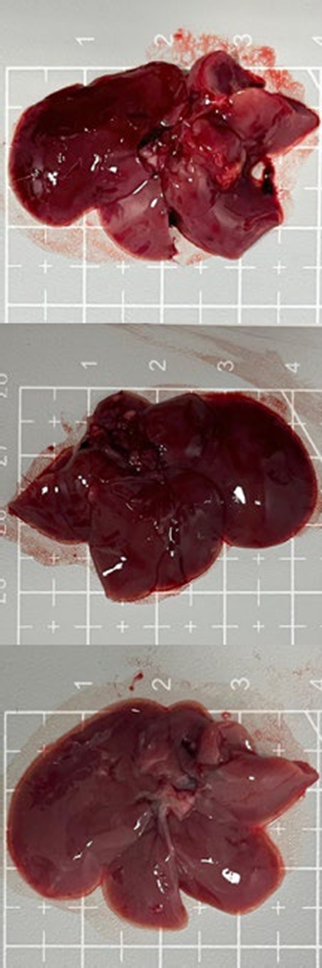
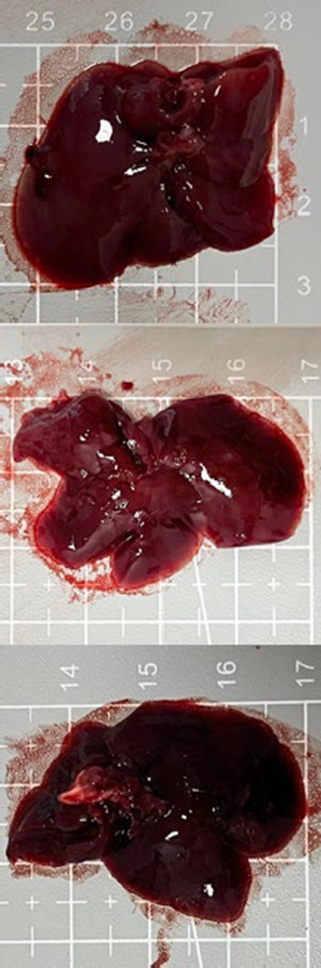
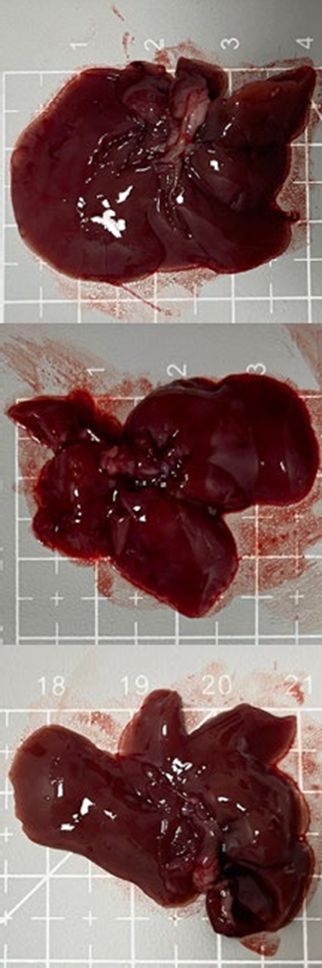
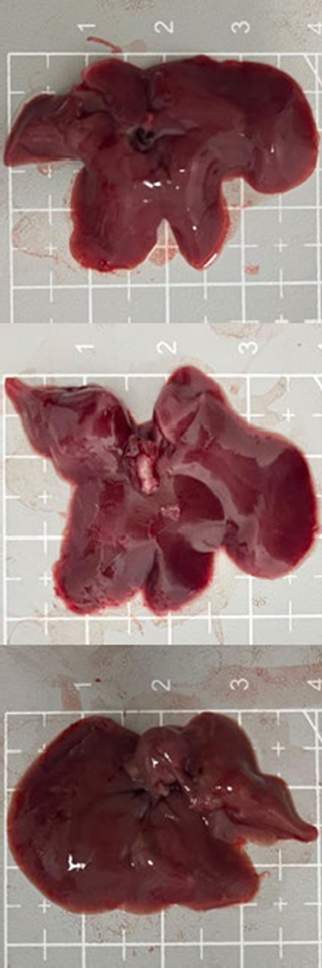
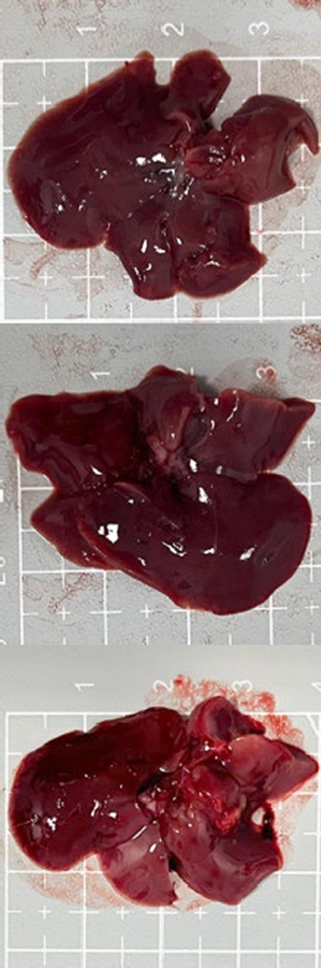
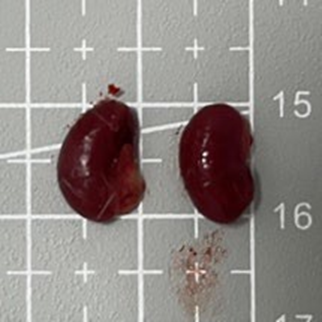
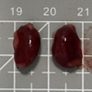
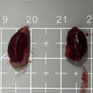
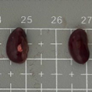
Tables 3 and 4 show no change in the color of the liver and kidneys of mice given avocado peel extract for 14 days.
The color of the liver and kidney organs in the control group and the test group have the same color of red with a chewy texture with no nodules on the surface.
Table 3. Effect of avocado peel extract on macroscopic liver of female mice before and after the treatment
|
|
|
|
|
|
|
Control |
1 |
2 |
3 |
4 |
|
1 |
|
|
|
|
|
2 |
|
|
|
|
|
3 |
|
|
|
|
Table 4. Effect of avocado peel extract on macroscopic kidneys of female mice before and after the treatment
|
|
|
Groups |
|
|
|
Control |
1 |
2 |
3 |
4 |
|
|
|
|
|
|
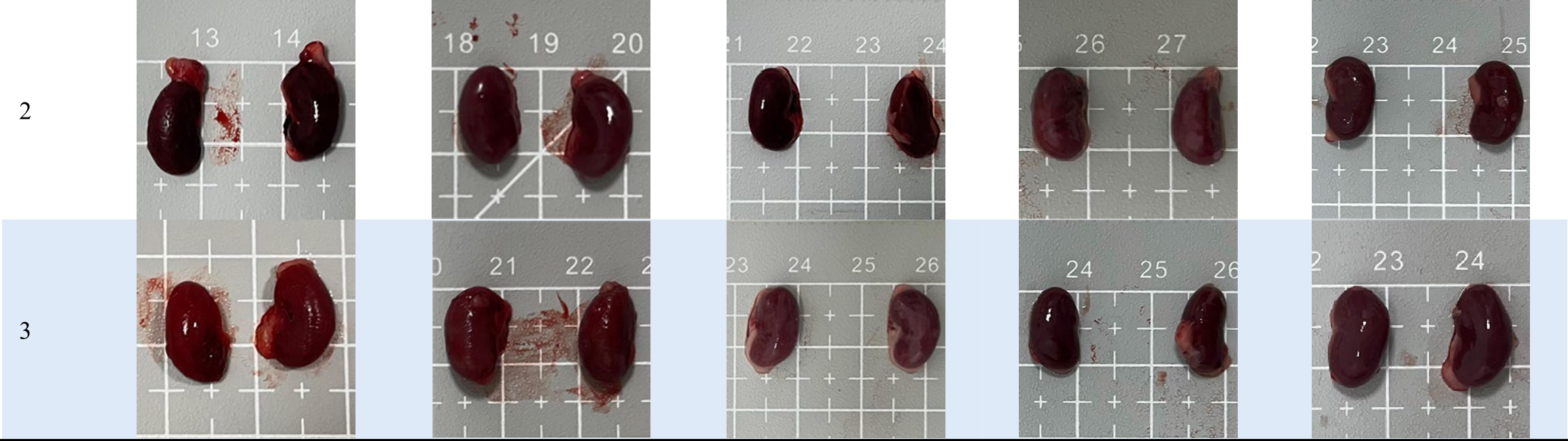
Table 5 shows the results of data processing using the Kruskal Wallis and ANOVA tests, the liver has a P-value of 0.661, and the kidney organ has a p value of 0.556. The data shows no significant difference (P>0.05) between the average organ size of the control group and the test group.
Table 6 illustrates that the relative organ weight of the liver and kidneys in the group given avocado peel ethanol extract showed no significant difference (P>0.05). This indicates that the administration of the extract did not affect the relative organ weights of the liver and kidneys. Therefore, the ethanol extract of avocado peel can be considered to have no significant effect on the relative organ weight of the liver and kidneys.
|
|||||||||||||||||||||||||||||||||||
|
Organ |
Average relative organ weight (grams) ± SD and ANOVA Test |
|||||
|
CG |
G1 |
G2 |
G3 |
G4 |
P-value |
|
|
Liver |
7.13 ± 1.94 |
6.63 ± 1.01 |
5.45 ± 0.68 |
7.18 ± 2.71 |
6.33 ± 1.13 |
0.710 |
|
Kidneys |
0.84 ± 0.14 |
0.80 ± 0.05 |
0.68 ± 0.14 |
0.88 ± 0.33 |
0.83 ± 0.23 |
0.777 |
|
Note: Control Group (CG), 300 mg/KgBW single dose (G1), 300 mg/KgBW repeat dose (G2), 2000 mg/KgBW single dose (G3), and 2000 mg/KgBW repeat dose (G4) Table 7. Classification categories according GHS toxicity test Group |
||||||
|
|||||||||||||
The peel extracts of P. americana were shown to be well tolerated by animals at an oral route dose of 2,000 mg/kg, based on the acute toxicity evaluation conducted on healthy female mice using the self-modified OECD 423 methodology. The mortality of the mice in this research was not observed. The number of deaths in this research can determine the test classification category of a material based on the GHS. The toxicity classification is presented in Table 5.
Death of mice may result from excessive intact compounds in the test material, such as phenolic compounds and alkaloids. Some secondary metabolites, including saponins and alkaloids, are thought to cause organ cell damage due to their slow excretion time. This allows them to have a longer duration of contact with liver cells, increasing the risk of damage to the organ [22]. Meanwhile, consumption of high concentrations of alkaloids can cause
acute poisoning in adult animals. Alkaloids have the ability to desensitize nicotinic acetylcholine receptors (nAChRs). Initially, alkaloids stimulate these receptors; however, continued exposure leads to receptor desensitization and inhibition of cation conductance (Na⁺, Ca²⁺, and K⁺). These effects occur at nAChRs in the neuromuscular junction, causing transient skeletal muscle fasciculation, which then progresses to paralysis and eventually leads to respiratory failure [23]. In addition, very high doses of flavonoids can trigger oxidative stress leading to liver and kidney damage [24]. Organ damage caused by these compounds can be a major factor leading to death; however, in this research, this mechanism did not occur, so no mortality was observed.
Furthermore, a decrease in weight among the mice was noted (Table 1). Although the results of the statistical analysis showed no significant change in body weight
(P>0,05). A change in body weight of the test animals is an indication that they may be sick, specifically when the body weight has decreased by 20% to 25% over a period of 7 days or more [25].The weight loss in mice can occur because the tannin compounds contained in avocado peel can cause protein precipitation, forming a mucous membrane on the surface of the small intestine. This mucous membrane then protects the small intestine, leading to absorption inhibition. Indirectly, this process affects the weight loss in mice [26]. This is consistent with the energy balance theory, which holds that regulating food intake is the major way to maintain body weight [27]. The likelihood of gaining weight increases with the amount of digestive contents that the small intestine absorbs.
In addition, the weight loss in mice can also be influenced by the stress condition of the mice due to the administration of the preparation, which then causes a decrease in appetite, leading to weight loss in the mice [28]. Stress can also be influenced by external factors, such as temperature, cage, activity, and food which then collaborate with internal factors genes as determinants of traits derived from parents and hormonal factors that regulate all body functions. In addition to stress, decreased appetite in mice can also be caused by flavonoids and alkaloids, which can act as stomach poisoning or stomach poison. When these compounds enter the body, the mice's digestive system is disrupted, causing them not to want to eat [25].
The behavioral changes observed (Table 2) showed no difference in the motor activity of all experimental groups compared to the control group. All mice did not show the Straub phenomenon, a condition where the mice's tails stand upright or form the letter “S”, indicating that there are no toxic symptoms in the neuromuscular system of the mice. This study also showed no piloerection, a condition in which the mice's fur becomes erect. This indicates that there are no toxic symptoms on the skin of the mice. Symptoms of ptosis, a condition in which the eyelids cover at least 50% of its eyes, did not occur in the mice, indicating no toxic symptoms in the eye organs [19,20].
The pineal reflex is observed by tickling the back of the mice's ears using the edge of a cotton bud. The corneal reflex is performed to determine whether or not there is a reflex when the edge of a cotton bud touches the mice's eyes. Flexion and Haffner are the reflex reactions of mice when the legs and base of the tail are flanked by tweezers. The response of turning away or squeaking is a normal form of reaction. Observations of these four reflexes showed no abnormalities in any of the test groups, indicating that there were no toxic symptoms in the sensory nervous system of the mice [19].
There was no abnormalities observed in all mice during hanging and reestablishment. This can be seen when the mice are able to hang on a horizontally elongated wire without falling and can climb up the wire within five seconds after hanging. These observations indicate that the ethanol extract of avocado peel does not cause pharmacological effects in the form of sedation and muscle relaxation effects, so that mice can hang and climb the wire. Observations for body posture were tested by looking at the speed of the mice's response to turning their bodies back to normal after being stretched out. The results showed that all mice were able to perform normal body postures [19,20].
Tremor is a response given by mice in the form of shaking their entire bodies. The results showed that none of the mice experienced tremors. The absence of tremors indicates that there are no toxic symptoms to the central nervous system of the mice. The administration of the avocado peel extract to mice also did not cause toxic symptoms in their digestive system, concluded from the normal excretion of faeces in all the test groups and the control group (black/dark brown-colored faeces and not too dense or liquid faeces) [20].
In addition, none of the mice experienced lacrimation and salivation. Lacrimation was assessed by the presence of tear discharge, judging by the presence of wetness around the eyes of the mice. The absence of abnormalities in lacrimation and salivation indicates that there were no toxic symptoms to the autonomic nervous system of mice [19,20].
Macroscopic observations in this research were made by examining whether there was a difference in color and texture between the test group and the control group. It can be said that there are macroscopic changes in the liver and kidneys if the color changes to pale yellow, the texture becomes hard, and nodules are usually present on the surface of the organs [29]. Liver damage caused by toxic substances result from the continuous and prolonged damage to hepatocyte cells. The dosage received by the organ can also influence the number of damaged hepatocyte cells, which may even lead to cell necrosis [30]. Furthermore, the accumulation of toxic substances in the body can cause an increase in filtration in the glomerulus. Glomerular damage may lead to glomerular thickening due to the process of cell proliferation. If the damage continues, kidney failure will occur, ultimately resulting in cell death. In this research (table 3 and 4) showed no difference in organ color between the control group and the test group.
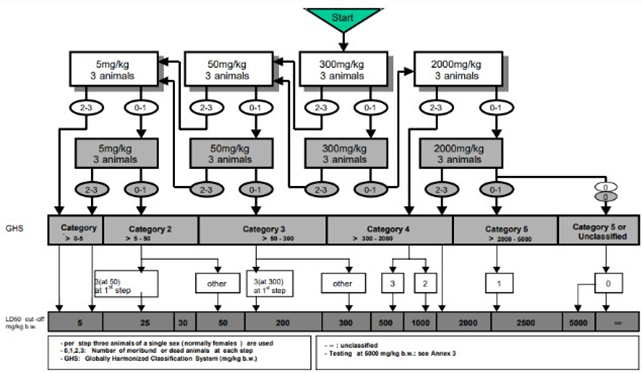
Figure 1. Test procedure of OECD 423
Differences in organ size in this research can be measured based on the length, width, and thickness of the organ. It can be said that there is a difference in organ size in the test group if there is a difference in size of 2-4 times that of the control group. The difference in organ size must also be accompanied by a significant change in relative organ weight. The size and weight of different relative organs can occur due to about 80% of the blood supply to the liver comes from the portal vein, making the liver a major organ vulnerable to exposure to toxic compounds in the body. The kidneys receive 25% of the cardiac output, which makes them susceptible to exposure to large amounts of chemicals because the process of removing metabolites in the kidneys can cause toxicity and tissue damage. Organ damage due to toxic substances is influenced by various factors, such as the type of chemical compound, the amount of dose given, and how long the exposure is received, whether acute, subchronic, or chronic. The higher the concentration of the compound, the greater the toxic response. Liver and kidney damage can occur quickly or slowly, over a matter of weeks to months [29–31].
Continuous and prolonged exposure of the liver to drugs and chemicals will cause changes in hepatocyte cells. Hepatocyte cells will experience fatty degeneration or necrosis, making it difficult for the hepatic cells to regenerate. The result of the accumulation of fat cells will cause enlargement of the liver, while prolonged damage to hepatocyte cells can cause permanent cell damage and can end in cell death. The kidneys can be damaged by toxic substances carried by the portal vein. Damage to the kidneys related to toxic substances usually occurs due to damaged glomeruli. Severely damaged glomeruli can cause toxic substances to flow into the renal tubules. Tubules that are severely damaged due to high intraglomerular pressure will cause glomerular atrophy. In this research, no significant difference was seen in the relative organ weight of the liver and kidneys in the control and the treated groups. The results (tables 3 and 4) showed no signs of toxicity effects in the form of changes in relative organ weight.
The results of this research are similar to the research by Mamadou et al., 2016, which used the Acute Toxic Class method, as performed in this research. The research conducted an acute toxicity test of ethanol extract from avocado leaves on Wistar rats with a dosage of 2,000 mg/kgBW for 14 days. The research results showed no rat deaths or toxic symptoms, including changes in behavior, during the observation period. Moreover, statistical analysis using ANOVA indicated no significant differences in body weight and relative liver and kidney weights between the treatment and control groups, although a non-significant increase in body weight was observed at the end of the study [32]. Another research tested the acute toxicity of an ethanol extract from avocado seeds on white rats. After 24 h, the highest dose of 5,000 mg/kgBW did not cause any deaths in the mice. This shows that the ethanol extract from avocado seeds is not toxic [33].
Avocado peel contains phenolic compounds, such as alkaloids, saponins, tannins, and flavonoids, which are also found in the leaves and seeds of the avocado. Although these phenolic compounds are present in all three parts of the plant, their concentrations vary among these parts. It is known that avocado peel contains more phenolic compounds compared to avocado leaves and
seeds. The phenolic compound content in avocado peel is 21.833 mg, while in avocado leaves it is 10.72 mg, and in avocado seeds it is 2.14 mg.
Conclusions
The administration of ethanol extract of avocado peel in mice at doses of 300 mg/KgBW and 2,000 mg/KgBW did not cause death, did not cause toxic symptoms in the form of behavioral changes, and did not cause macroscopic changes in organs. The effect on body weight was a decrease in body weight; however, the weight loss observed in each treatment group showed a decrease that is almost the same, without a significant difference to the control group. Therefore, avocado peel extract is not toxic. The above conclusions suggest that the public should be mindful of the dosage when using avocado peel ethanol extract, as it can significantly impact body weight. Furthermore, further research is required to be conducted on sub-chronic toxicity tests to identify potential toxic effects.
Conflict of Interests
The authors have no conflict of interest related to this study.
Funding
This research was not supported by any grants from public or commercial and was entirely funded by the author’s personal funds.
Acknowledgement
The authors are grateful to their supervisor, for the insightful advice and to the laboratory staff of the Department of Biochemistry, Department of Pharmacology, and Department of animal research of the Faculty of Medicine Jenderal Achmad Yani University for their kind support during the experimental work. This article is part of an ongoing research project conducted as requirement for the undergraduate thesis at Jenderal Achmad Yani University.
Compliance with Ethical Guidelines
The research adhered to all applicable ethical guidelines for the use of animals in research. Ethical clearance was obtained from the Health Research Ethics Committee Faculty of Medicine, Jenderal Achmad Yani University, on October 4, 2024 (Approval: 017/UH1.10/2024)
Authors' Contributions
Welly Ratwita, Fransiska Ambarukmi Pontjosudargo, Evy Shavilla, and Aprillia Grace Sweetasari were involved in designing the concept, supervising, giving direction during the experiment, and revising during the manuscript development. Afni Yuafi Mayzida, Aninditha Larasati Dewi, Nafisa Silmi Kaffah, Nurfadhiya Previ Maharani were contributed to conducting experiments from data collection to analyzing and interpreting the data. All authors contributed to the discussion and revision the manuscript.
References
1. Hartati S, Yunus A, Nandariyah N, Yuniastuti E, Pujiasmanto B, Purwanto E, et al. Diversifikasi tanaman pekarangan dengan tanaman alpukat untuk meningkatkan gizi keluarga. Semar (Jurnal Ilmu Pengetahuan, Teknologi, Dan Seni Bagi Masyarakat).2022;
2. Rotta EM, de Morais DR, Biondo PBF, dos Santos VJ, Matsushita M, Visentainer JV. Use of avocado peel (Persea americana) in tea formulation: A functional product containing phenolic compounds with antioxidant activity. Acta Scientiarum – Technology. 2016;38(1): 23–29. [doi:10.4025/actascitechnol.v38i1.27397]
3. Salam A. Bahan ajar herbal medicine (Vol. 1). PT. Literasi Nusantara Abadi Grup. 2023. [Link]
4. Akan S. Phytochemicals in avocado peel and their potential uses. Food and Health. 2021;7(2): 138–149. [doi:10.3153/FH21015]
5. Jiménez Patiño P, Garcia Concha P, Quitral V, Vasquez K, Parra- Ruiz C, Reyes-Farias M, et al. Pulp, leaf, peel and seed of avocado fruit: A review of bioactive compounds and healthy benefits. Food Rev Int. 2021;37(6):619–655). [doi:10.1080/87559129.2020.1717520]
6. Mendrofa EPM, Halawa C, Fachrial E, Lubis YM. In vitro antifungal effectiveness of avocado peel extract (Persea americana) Candida albicans and Aspergillus niger. Jurnal Biosains. 2019;5(1). [doi:10.24114/jbio.v5i1.12378]
7. Lyu X, Agar OT, Barrow CJ, Dunshea FR, Suleria HAR. Phenolic compounds profiling and their antioxidant capacity in the peel, pulp, and seed of Australian grown avocado. Antioxidants (Basel). 2023;12(1):185. [doi: 10.3390/antiox12010185] [pmid: 36671046]
8. Ferreira SM, Falé Z, Santos L. Sustainability in skin care: Incorporation of avocado peel extracts in topical formulations. Molecules. 2022;27(6):1782.
[doi: 10.3390/molecules27061782] [pmid: 35335146]
9. Armayanti I, Wardani MK, Yosi A. In vitro effectiveness of the avocado peel extract (Persea americana Mill) as an antifungal against Trichophyton rubrum, the cause of dermatophytosis. Med Glas (Zenica). 2023;20(2). [doi: 10.17392/1587-23] [pmid: 37300472]
10. Organisation for Economic Co-operation and Development. OECD guideline for testing of chemicals: Acute oral toxicity— Acute toxic class method (OECD/OCDE 423). 2001. [Link]
11. Biologi J, Sains dan Teknologi F, Alauddin Makassar U, Hasanah U, Masri M, Alauddin Makassar JI. Prosiding seminar nasional mikrobiologi Kesehatan dan lingkungan. Makassar. 2015;29. [Link]
12. Aprilianto E, Harmoni Swantika Yuan AV, Pradita CD, Hendra
P. Anti-inflammatory effects of avocado peels against inflammation induced by carrageenan in mice. Pharmaciana. 2019;9(2):222–224. [doi:10.12928/pharmaciana.v9i2.13607]
13. Rahman N, Sabang SM, Abdullah R, Bohari B. Antioxidant properties of the methanolic extract of avocado fruit peel (Persea americana Mill.) from Indonesia. J Adv Pharm Technol Res. 2022;13(3):166-170. [doi: 10.4103/japtr.japtr_22_22]
14. Bamidele TO., Ubana MA, Emmanuel V, Oyedeji TA. Phytochemical constituents, nutritional and anti-nutritional composition of Persea americana seeds. Savanna J Basic Appl Sci. 2021; 3(2):117-123. [Link]
15. Cincin Kirana B, Fatmadewi Imawati M, Aprilia AE. Perbandingan kadar flavonoid ekstrak etanol daun dan kulit buah alpukat (Persea americana Mill). Jurnal Kesehatan Jompa. 2023;2(2). [doi: 10.57218/jkj.Vol2.Iss2.915]
16. Indonesia, Badan Pengawas Obat dan Makanan. Badan Pengawas Obat dan Makanan Peraturan nomor 10 tahun 2022 pedoman uji toksisitas praklinik secara in vivo. 2022. [Link]
17. Yuslianti ER, Dewi ZY, Al-wasi DM. Uji toksisitas akut disclosing solutions buah naga super merah (Hylocereus costaricensis) freeze dry: Studi eksperimental. Padjadjaran J Den Res Students. 2024; 8(1):59. [doi:10.24198/pjdrs.v8i1.53195]
18. Gunanegar RF, Reni Yuslianti E, Anshori K, Sunarti S. Pengaruh pemberian akut kacang tanah Bambara (Vigna subterranea) terhadap mortalitas, bobot badan, gejala toksisitas, dan bobot organ mencit Balb-C betina: Kajian uji toksisitas akut. Medika Kartika Jurnal Kedokteran Dan Kesehatan. 2023;6(3):295–306.[doi:10.35990/mk.v6n3.p295-306]
19. Yuslianti ER, Meliawaty F, Sutjiatmo AB, Pradita A. Acute toxicity test of rambutan honey adhesive cream against Swiss Webster mice (Uji toksisitas akut krim perekat madu rambutan terhadap mencit Swiss Webster). Special Issues Smart Dentistry. 2022. [doi:10.54052/jhds.sd22.p133-144]
20. Sujana D, Winda Suwandi D, Rusdiana T, Subarnas A. Acute toxicity test of ethanol extract of pakis tangkur (Polypodium feei Meet) root from Talaga Bodas Mountain on Swiss Webster mice. Jurnal Ilmiah Farmako Bahari. 2022:167-179. [Link]
21. Globally Harmonized System. Globally harmonized system of classification and labelling of chemicals (GHS). 2023. [Link]
22. Parwanty GM, Azizah N, Darotulmutmainah A, Stikes F, Kuningan
M. Uji toksisitas akut ekstrak etanol anggur merah (Vitis vinifera Linn) menggunakan metode Thomson dan Weil pada tikus putih Wistar (Rattus norvegicus). Jurnal Ilmu Farmasi. 2023;14(1). [doi: 10.61902/cerata.v14i1.759]
23. Green BT, Lee ST, Panter KE, Brown DR. Piperidine alkaloids: Human and food animal teratogens. Food Chem Toxicol. 2012;50(6):2049- 2055. [doi: 10.1016/j.fct.2012.03.049] [pmid: 22449544]
24. Tang Z, Zhang Q. The potential toxic side effects of flavonoids. Biocell. 2021;46(2):357–366. [doi:10.32604/biocell.2022.015958]
25. Djamaludin M, Kristiana R, Permana BY. Acute toxicity test of ethanol extract bay leaf (Syzygium polyanthum) against mice againts mice (Mus musculus) DDY strain. Kartika Medika: Jurnal Kedokteran dan Kesehatan. 2021 Sep;4(4):356–65.
26. Sinulingga AS. Penurunan berat badan mencit Swiss-Webster betina pada pemberian infusa daun asam jawa (Tamarindus indica L.). Farmasis: Jurnal Sains Farmasi. 2023; 4(1): 1–6. [doi:10.36456/farmasis.v4i1.6997]
27. Sherwood L. Introduction to human physiology (8th ed.). Cengage Learning. 2013. [Link]
28. Putri CPM, Ansory HM, Hanifah IR. Uji toksisitas akut miristisin terhadap mencit putih betina (Mus musculus). Majalah Farmaseutik. 2024;20(2):132.
[doi:10.22146/farmaseutik.v20i2.81477]
29. Ceriana R, Putri Rejeki D. Uji toksisitas makroskopis pada organ ginjal, hati, jantung, dan limpa mencit hiperglikemia yang diberi ekstrak etanol kulit buah rambai (Baccaurea motleyana). Journal of Pharmaceutical and Health Research. 2023;4(2):183–189. [doi:10.47065/jharma.v4i2.3366]
30. Ceriana R, Putri NZ. Degenerasi dan nekrosis pada hati mencit diabetes yang diberi ekstrak kulit buah rambai (Baccaurea motleyana Muell. Arg). Journal of Healthcare Technology and Medicine. 2019;4(1):103. [doi:10.33143/jhtm.v4i1.172]
31. Widyaningsih A, Sitasiwi AJ, Mardiati SM. Respon glomerulus ren mencit (Mus musculus L.) terhadap pemberian senyawa antifertilitas dari ekstrak air biji pepaya (Carica papaya L.). Buletin Anatomi Dan Fisiologi. 2019;3(2):233–241. [doi:10.14710/baf.3.2.2018.233-241]
32. Mamadou K, N’Goran MK, Eugene K, Amani BK, Koffi C, N’Guessan ARY, et al. Acute toxicity and hypoglycaemic activity of the leaf extracts of Persea americana Mill. (Lauraceae) in Wistar rats. African J Pharm Pharmacol. 2016;10(33):690– 698. [doi:10.5897/ajpp2016.4617]
33. Nwachukwu CM, Emerenini NJ, Egege JN, Ogburubi O. Toxicological studies of the effects of ethanolic extracts of avocado (Persea americana Mill.) seed flour on experimental animals. In J Sci Res Arch. 2023;10(2):946–954. [doi:10.30574/ijsra.2023.10.2.1016]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |