Introduction
Aluminum phosphide (AlP) is a solid fumigant widely used since the 1940s and is readily available [1]. In Iran, this combination is known as rice tablets, which can be purchased in local stores [2]. This solid pesticide quickly became one of the most used seed fumigants and is considered an ideal drop killer due to its properties [3]. This substance has become a common cause of poisoning in Iran [4, 5] and is one of the leading causes of suicide in many countries [4, 6]. Poisoning caused by rice tablets is due to the release of phosphine gas [7]. This chemical is typically formulated as pills, granules, or powder and is extremely poisonous and inexpensive. One of its activators, phosphine gas, is created due to a chemical reaction that occurs when it interacts with the humidity of the surroundings [7]. A relatively tiny amount of AlP tablets causes the emission of phosphine gas, which affects a variety of organs [8]; the heart, digestive system, lungs, and kidneys are the primary organs that are affected by the initial exposure.
Cells undergo an energy crisis as a result of phosphine's non-competitive inhibition of mitochondrial cytochrome oxidase, blockage of the electron transport chain, and oxidative phosphorylation [6]. Phosphonate can immediately disturb the mitochondrial environment by impairing the cytochrome c enzyme's ability to operate. It may also significantly reduce the mitochondrial membrane potential and diminish oxidative respiration by 81% [9]. This poisoning also frequently results in metabolic acidosis, which is carried on by the accumulation of lactic acid brought on by the oxidative phosphorylation block, which results in inadequate blood flow to the tissues [10]. In order to treat AlP poisoning properly, phosphine absorption and cytotoxicity should be reduced and excretion through the kidneys and lungs should increase [11]. The usual fatal dosage of AlP is between 0.15 and 0.5 grams, and the risk of poisoning with this medication is relatively high [4, 5, 12]. However, there is not a precise remedy for poisoning from rice pills.
There is no proven antidote that has been clinically proven to work [13]. Most AlP toxicity treatments are supportive in nature, such as intra-aortic balloon pumps [14] and extracorporeal membrane oxygenation (ECMO), a recently developed and considerably promising method that offers transient cardiorespiratory support [15-17]. Magnesium sulfate [18-20], melatonin [21], coconut oil [22, 23], acetylcysteine (NAC) [24-27], sodium selenite [28], vitamin E [29, 30], triiodothyronine [31], liothyronine [32], vasopressin and milrinone [31], Laurus nobilis L [33], Aminonicotinamide (6-AN), boric acid [34, 35] and acetyl L-carnitine are among the antidotes investigated in different studies [36]. However, none of these antidotes have been used in clinical settings.
The data offered and the research on potential antidotes suggest that further study is still needed to develop effective antidotes. The impact of AlP poisoning at various dosages as well as the chemicals sodium bromide (NaBr), calcium carbonate (CaCO3), and potassium nitrate (KNO3), have been investigated in the current study as potential antidotes on ovarian and digestive system tissues. This is because rice tablets are one of the most frequent causes of acute poisoning and suicide in Iran, and neither the mechanism of action nor the antidote for them has been fully elucidated.
Materials and Methods
Study design
The present study was an in vivo study that was conducted on female Wistar rats poisoned with AlP tablets. This study was performed in compliance with the relevant regulations and institutional guidelines and has been approved by the ethics committee of Tabriz Medical School, Iran, with the ethics code IR.TBZMED.VCR.REC.1399.353. All the ethical principles of working with animals were considered by the researchers. The present study was conducted in two parts. In the first part, the toxicity of different concentrations of AlP was investigated on female rats, and in the second stage, different concentrations of antidotes were investigated.
Toxicity with different concentrations of AlP
In the first part of the study, 36 female Wistar rats were divided into six groups. AlP tablets with different concentrations (0-40 mg/kg) were fed to them. After 24 h of AlP administration, the rats were bled and euthanized under anesthesia, and the tissues of the digestive system (esophagus, stomach, duodenum, and pancreas) as well as the uterus and ovaries were removed and placed in 10% formalin. A block was taken, and a cross-section was prepared; then, hematoxylin and eosin staining were done to carry out histopathological examination.
Therefore, two rats died in the AlP concentration of 40 mg /kg; the AlP concentration for the next phase was equal to 20 mg/kg.
Effect of different concentrations of antidotes on AlP toxicity
A total of 66 female Wistar rats with an approximate weight of 200-250 grams were divided into 11 groups, as presented in Table 1.
Table 1. Prepared groups of treated rats with AlP and antidots
| Group |
Treatment |
| Group 1 |
Control without intervention |
| Group 2 |
AlP - 40 mg/kg body weight as a single dose gavage |
| Group 3 |
AlP - 40 and after 20 min low dose NaBr |
| Group 4 |
AlP - 40 mg/kg body weight and after 20 min high dose NaBr |
| Group 5 |
AlP - of 40 and after 20 min CaCO3 with a low dose by gavage |
| Group 6 |
AlP - 40 and after 20 min CaCO3 with a high dose |
| Group 7 |
AlP - 40 and after 20 min KNO3 with a low dose |
| Group 8 |
AlP - 40 and after 20 min KNO3 with a high dose |
| Group 9 |
High dose NaBr |
| Group 10 |
High dose CaCO3 |
| Group 11 |
High dose KNO3 |
After 24 h, fertility hormones, including follicle-stimulating hormone (FSH) and luteinizing hormone (LH), were measured using the enzyme-linked immunosorbent assay (ELISA) technique.
Tissue sampling for histopathology studies of esophagus, stomach, pancreas, uterus, and ovary
Tissue samples from the esophagus, stomach, pancreas, uterus, and ovary were fixed in 10% buffered formalin. After 24 h, thin sections of the tissues were prepared for tissue processing. The tissues were dehydrated using alcohol, and then a clarification step was performed using xylol (Tissue Processor Model DS 2080/H, Did Sabz CO., Iran). Finally, the impregnation and molding stages were performed using melted paraffin. After preparing 4–5-micron slices from the molds with a microtome device, they were placed in a 56°C oven for 30 min, and after cooling, the usual hematoxylin and eosin staining was performed.
Evaluation of tissue sections
Prepared slides were examined by a conventional light microscope (OLYMPUS-CH30, Japan) and according to Klopfleisch’s [37] study for vascular hyperemia, edema, hemorrhage, amas, and necrosis factors. In all studied cases, in the absence of the desired lesion, the normal state (N), a mild lesion as +1, a moderate lesion as +2, and a severe lesion as +3 were considered.
Data analysis
Statistical analysis of the obtained results was carried out using ANOVA, or Kruskal-Wallis H and Mann-Whitney U tests for parametric and non-parametric data, respectively, using the SPSS (version 22) software. In addition, Pearson's test was employed to investigate the relationship between different parameters.
Results
Histopathological studies of the esophagus, stomach, pancreas, and intestine
The results of the microscopic examination of the examined tissue sections are indicated in Figures 1–4. Esophageal tissue in all groups showed normal tissue structure, and no pathological lesions were observed in the tissue sections studied in the control group and other experimental groups in the squamous epithelium and submucosa of the esophagus (Figure 1).
Pancreatic tissue in all groups showed normal tissue structure, and no pathological lesions were observed in the tissue sections studied in the control group and other test groups in pancreatic acini and islets of Langerhans (Figure 2).
In the stomach tissue of the control group, normal tissue structure was observed (Figure 3). Only in the AlP-4 and AlP-5 groups, there was a mild gastritis with mononuclear mast cells and a small number of eosinophils under the gastric mucosa, which was not significantly different from the other groups. In other test groups, almost normal tissue structure was observed in the epithelium, gastric glands, and submucosa. There were no other pathological lesions, including edema, hyperemia, hemorrhage, and necrosis.
In the intestinal tissue of the control group, a completely normal tissue structure was observed (Figure 4). In the other test groups, there was mild mononuclear enteritis in the mucosa and submucosa of the intestinal tissue, and no significant difference was observed between the groups. There were no other pathological lesions, including edema, hyperemia, hemorrhage, and necrosis. In addition, the epithelium and glands showed normal structures.
In the control group, the normal structure of uterine and ovarian tissue was observed. Moreover, in other studied groups, common pathological lesions, including vascular hyperemia, edema, bleeding, amas, and necrosis, were not observed, and almost normal structure was seen in all tissue sections (Figure 5).
In the uterine tissue, the normal structure of the epithelium covering the endometrium and uterine glands was observed. In the ovarian tissue, there were active ovarian follicles in different stages of development (Figure 6).
Relationship between the studied parameters and AlP concentration
Pearson's correlation was used to investigate the relationship between the studied hormones and AlP concentration, and the results are indicated in Table 2. Luteinizing hormone (LH), progesterone, and estradiol were found to be inversely related to AlP (P<0.05). However, there was no significant relationship between AlP concentration and FSH or prolactin levels (P=0.728).
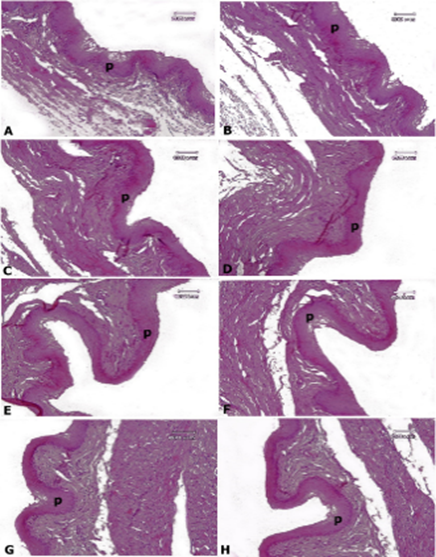
Figure 1. Rat esophagus tissue, A: control group, B: AlP-5 group, C: AlP-4 group, D: A2 group, E: B2 group, F: C2 group, G: AD1, H: AD3. In all groups, an almost normal structure with a fatty esophagus and a normal squamous epithelium (p) is observed—Hematoxylin-eosin staining.
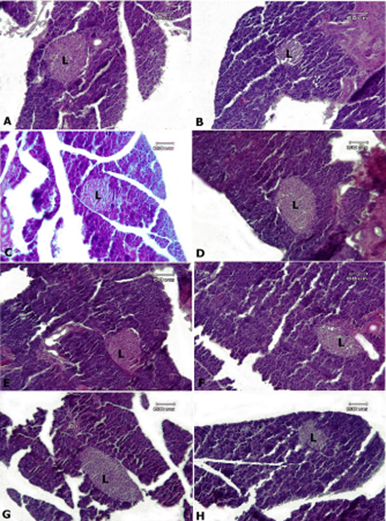
Figure 2. Rat pancreas tissue, A: control group, B: AlP-5 group, C: AlP-4 group, D: A2 group, E: B2 group, F: C2 group, G: AD1, H: AD3. In all groups, normal structure is observed in pancreatic acini and islets of Langerhans (L)—Hematoxylin-eosin staining.
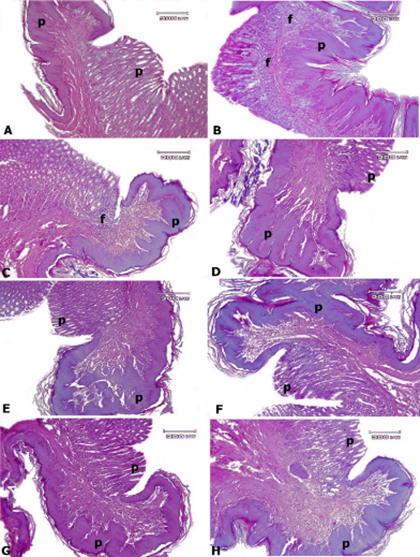
Figure 3. Rat stomach tissue, A: control group, B: AlP-5 group, C: AlP-4 group, D: A2 group, E: B2 group, F: C2 group, G: AD1, H: AD3. In the control group, a normal structure is observed in the mucosa (P) and submucosa of the stomach tissue. Only in the AlP-4 and AlP-5 groups, there was mild gastritis (f) in the submucosa of the stomach. In other test groups, almost normal tissue structure was observed in the epithelium, gastric glands, and submucosa—Hematoxylin-eosin staining.
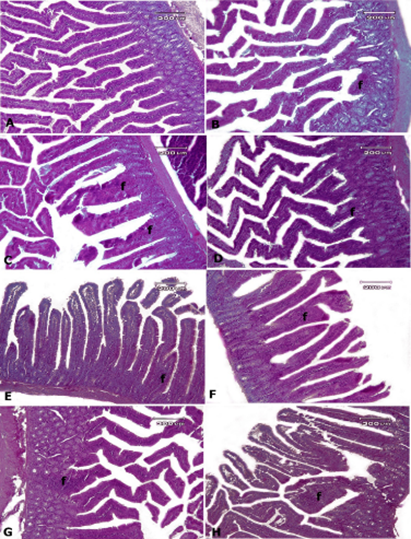
Figure 4. Rat intestinal tissue, A: control group, B: AlP-5 group, C: AlP-4 group, D: A2 group, E: B2 group, F: C2 group, G: AD1, H: AD3. In the control group, normal structure is observed in the intestinal tissue. In the other test groups, mild mononuclear enteritis (f) was present in the mucosa and submucosa of the intestinal tissue—Hematoxylin-eosin staining.
Levels of the hormones as a result of poisoning with AlP and treatment with antidotes
Rats poisoned with AlP were treated with different antidotes. The effect of the studied antidotes is presented in Table 3. According to the obtained results, the levels of all the examined hormones, including FSH (P<0.0001), LH (P=0.001), prolactin (P=0.002), testosterone (P <0.0001), progesterone (P<0.0001), and estradiol (P<0.0001), had significant differences between different treatments.
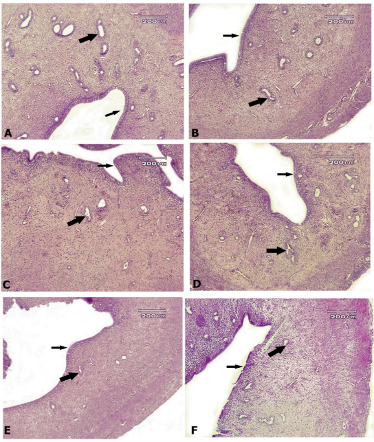
Figure 5. Rat uterine tissue, A: control group, B: AlP-5 group, C: AlP-4 group, D: A2 group, E: B2 group, F: C2 group. In all groups, an almost normal structure is observed, with uterine fat in the normal epithelium (small arrows) and uterine glands (big arrows)—Hematoxylin-eosin staining.
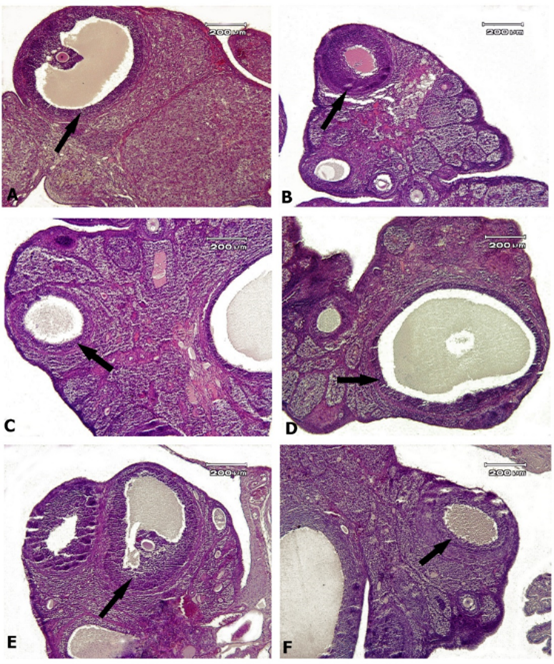
Figure 6. Rat ovary tissue, A: control group, B: AlP-5 group, C: AlP-4 group, D: A2 group, E: B2 group, F: C2 group. An almost normal structure with normal ovarian fat and active ovarian follicles (arrows) is observed in all groups—Hematoxylin-eosin staining.
| Correlation to AlP |
FSH |
LH |
Prolactin |
Progesterone |
Estradiol |
| Pearson Correlation |
-0.066 |
-0.553 |
-0.224 |
-0.751 |
-0.875 |
| P-value |
0.728 |
0.002 |
0.234 |
<0.0001 |
<0.0001 |
Table 3. Levels of the hormones as a result of poisoning with AlP and treatment with antidotes
| Group |
No |
Mean |
SD |
95% Confidence Interval for Mean |
P-value |
| Lower Bound |
Upper Bound |
| FSH |
AlP |
5 |
1.30 |
0.15 |
1.12 |
1.48 |
<0.0001 |
| AlP-KNO3 |
12 |
1.33 |
0.12 |
1.25 |
1.41 |
| AlP-CaCO3 |
12 |
1.88 |
0.58 |
1.51 |
2.25 |
| AlP-NaBr |
12 |
1.33 |
0.18 |
1.21 |
1.44 |
| Control |
6 |
1.33 |
0.33 |
0.99 |
1.68 |
| KNO3 |
6 |
1.12 |
0.15 |
0.96 |
1.27 |
| CaCO3 |
6 |
1.50 |
0.96 |
1.40 |
1.60 |
| NaBr |
6 |
1.32 |
0.15 |
1.15 |
1.48 |
| LH |
AlP |
5 |
0.60 |
0.57 |
-0.11 |
1.31 |
0.001 |
| AlP-KNO3 |
12 |
2.00 |
2.17 |
0.62 |
3.38 |
| AlP-CaCO3 |
12 |
3.46 |
1.84 |
2.29 |
4.62 |
| AlP-NaBr |
12 |
3.47 |
3.91 |
0.99 |
5.95 |
| Control |
6 |
0.90 |
0.50 |
0.38 |
1.42 |
| KNO3 |
6 |
1.73 |
1.22 |
0.45 |
3.01 |
| CaCO3 |
6 |
2.00 |
0.26 |
1.73 |
2.27 |
| NaBr |
6 |
6.63 |
3.75 |
2.70 |
10.57 |
| Prolactin |
AlP |
5 |
0.78 |
0.17 |
0.57 |
0.99 |
0.002 |
| AlP-KNO3 |
12 |
2.14 |
1.39 |
1.25 |
3.02 |
| AlP-CaCO3 |
12 |
1.47 |
0.40 |
1.21 |
1.72 |
| AlP-NaBr |
12 |
1.27 |
0.39 |
1.02 |
1.51 |
| Control |
6 |
3.53 |
3.17 |
0.21 |
6.86 |
| KNO3 |
6 |
0.70 |
0.18 |
0.51 |
0.89 |
| CaCO3 |
6 |
2.46 |
1.31 |
1.08 |
3.84 |
| NaBr |
6 |
1.55 |
0.65 |
0.87 |
2.23 |
| Testosterone |
AlP |
5 |
0.26 |
0.09 |
0.15 |
0.38 |
<0.0001 |
| AlP-KNO3 |
12 |
0.32 |
0.13 |
0.24 |
0.41 |
| AlP-CaCO3 |
12 |
0.16 |
0.06 |
0.12 |
0.20 |
| AlP-NaBr |
12 |
0.69 |
0.27 |
0.51 |
0.86 |
| Control |
6 |
0.53 |
0.30 |
0.50 |
0.56 |
| KNO3 |
6 |
. |
. |
. |
. |
| CaCO3 |
6 |
. |
. |
. |
. |
| NaBr |
6 |
. |
. |
. |
. |
| Progesterone |
AlP |
5 |
28.66 |
7.14 |
19.79 |
37.53 |
<0.0001 |
| AlP-KNO3 |
12 |
12.98 |
9.08 |
7.21 |
18.76 |
| AlP-CaCO3 |
12 |
32.72 |
20.55 |
19.66 |
45.77 |
| AlP-NaBr |
12 |
39.60 |
5.80 |
35.91 |
43.29 |
| Control |
6 |
48.20 |
1.59 |
46.53 |
49.87 |
| KNO3 |
6 |
38.50 |
15.34 |
22.40 |
54.60 |
| CaCO3 |
6 |
2.52 |
0.28 |
2.22 |
2.81 |
| NaBr |
6 |
31.60 |
22.63 |
7.85 |
55.34 |
| Estradiol |
AlP |
5 |
16.24 |
0.34 |
15.82 |
16.66 |
<0.0001 |
| AlP-KNO3 |
12 |
22.08 |
3.84 |
19.65 |
24.52 |
| AlP-CaCO3 |
12 |
15.70 |
2.03 |
14.41 |
16.99 |
| AlP-NaBr |
12 |
10.65 |
3.56 |
8.39 |
12.91 |
| Control |
6 |
8.63 |
2.64 |
5.86 |
11.41 |
| KNO3 |
6 |
16.52 |
1.64 |
14.80 |
18.24 |
| CaCO3 |
6 |
16.90 |
0.93 |
15.92 |
17.88 |
| NaBr |
6 |
10.25 |
1.54 |
8.64 |
11.86 |
Table 2. Relationship between the studied parameters and AlP concentration
According to the obtained results, the FSH hormone level in the AlP-CaCO3 group was significantly higher than that in the AlP (P=0.014), AlP-KNO3 (P=0.001), AlP-NaBr (P=0.001), normal control (P=0.014), KNO3 (P<0.0001) and NaBr (P=0.010) groups.
The LH hormone was higher in the NaBr group compared to that in the AlP (P=0.003), AlP-KNO3 (P=0.008), normal control (P=0.003), KNO3 (P=0.020), and CaCO3 (P=0.033) groups.
Prolactin hormone in the normal control group was significantly higher than that in the AlP (P=0.011), AlP-CaCO3 (P=0.028), AlP-NaBr (P=0.011), and KNO3 (P=0.004) groups.
Progesterone in the AlP-KNO3 group was significantly lower than that in the AlP-CaCO3 (P=0.012), AlP-NaBr (P<0.0001), normal control (P<0.0001) and KNO3 (P =0.006) groups. Moreover, progesterone hormone in the CaCO3 group was significantly lower than that in the rats receiving AIP (P=0.035), AlP-CaCO3 (P=0.001), AlP-NaBr (P<0.0001), normal control (P<0.0001), KNO3 (P>0.0001) and NaBr (P=0.007).
Regarding the estradiol hormone, the results demonstrated that the level of estradiol in rats receiving AlP was higher compared to that in the groups receiving AlP-NaBr (P=0.006), normal control (P<0.0001) and NaBr (P=0.011). On the other hand, it was found that the amount of estradiol in the group treated with AlP-KNO3 was higher compared to that in the groups receiving AlP (P=0.003), AlP-CaCO3 (P<0.0001), AlP-NaBr (P<0.0001), normal control (P<0.0001), KNO3 (P=0.003), CaCO3 (P=0.007) and NaBr (P<0.0001)
Levels of the examined hormones as a result of AlP poisoning and treatment with different doses of antidotes
Each antidote used in this study was used in two high and low doses. Table 4 shows the results of the evaluation of hormones after poisoning with AlP and treatment with antidotes in high and low doses. According to the results, the levels of all hormones were significantly different between the studied groups (P<0.0001).
Table 4. Levels of hormones investigated as a result of AlP poisoning and treatment with different doses of antidotes.
| Group |
No |
Mean |
SD |
95% Confidence Interval for Mean |
P-value |
| Lower Bound |
Upper Bound |
| FSH |
AlP |
6 |
1.31 |
0.14 |
1.17 |
1.46 |
<0.0001 |
| AlP-KNO3-L |
6 |
1.28 |
0.14 |
1.13 |
1.43 |
| AlP-KNO3-H |
6 |
1.38 |
0.09 |
1.29 |
1.47 |
| AlP-CaCO3-L |
6 |
2.25 |
0.59 |
1.63 |
2.89 |
| AlP-CaCO3-H |
6 |
1.50 |
0.23 |
1.26 |
1.74 |
| AlP-NaBr-L |
6 |
1.46 |
0.16 |
1.29 |
1.62 |
| AlP-NaBr-H |
6 |
1.20 |
0.10 |
1.10 |
1.30 |
| Control |
6 |
1.33 |
0.33 |
0.99 |
1.68 |
| KNO3-H |
6 |
1.12 |
0.15 |
0.96 |
1.27 |
| CaCO3-H |
6 |
1.50 |
0.10 |
1.40 |
1.60 |
| NaBr-H |
6 |
1.32 |
0.15 |
0.15 |
1.48 |
| LH |
AlP |
6 |
0.73 |
0.61 |
0.10 |
1.37 |
<0.0001 |
| AlP-KNO3-L |
6 |
0.40 |
0.21 |
0.18 |
0.62 |
| AlP-KNO3-H |
6 |
3.60 |
2.05 |
1.45 |
5.75 |
| AlP-CaCO3-L |
6 |
4.27 |
2.31 |
1.84 |
6.70 |
| AlP-CaCO3-H |
6 |
2.64 |
0.69 |
1.92 |
3.37 |
| AlP-NaBr-L |
6 |
6.00 |
4.23 |
1.56 |
10.44 |
| AlP-NaBr-H |
6 |
0.93 |
0.48 |
0.43 |
1.44 |
| Control |
6 |
0.90 |
0.50 |
0.38 |
1.42 |
| KNO3-H |
6 |
1.73 |
1.22 |
0.45 |
3.01 |
| CaCO3-H |
6 |
2.00 |
0.26 |
1.73 |
2.27 |
| NaBr-H |
6 |
6.63 |
3.75 |
2.70 |
10.57 |
| Prolactin |
AlP |
6 |
0.77 |
0.15 |
0.61 |
0.93 |
<0.0001 |
| AlP-KNO3-L |
6 |
3.34 |
0.89 |
2.41 |
4.27 |
| AlP-KNO3-H |
6 |
0.94 |
0.20 |
0.74 |
1.15 |
| AlP-CaCO3-L |
6 |
1.18 |
0.15 |
1.02 |
1.35 |
| AlP-CaCO3-H |
6 |
1.75 |
0.37 |
1.36 |
2.14 |
| AlP-NaBr-L |
6 |
1.35 |
0.31 |
1.02 |
1.68 |
| AlP-NaBr-H |
6 |
1.18 |
0.47 |
0.69 |
1.68 |
| Control |
6 |
3.53 |
3.17 |
0.21 |
6.86 |
| KNO3-H |
6 |
0.70 |
0.18 |
0.51 |
0.89 |
| CaCO3-H |
6 |
2.46 |
1.31 |
1.08 |
3.84 |
| NaBr-H |
6 |
1.55 |
0.65 |
0.87 |
2.23 |
| Testosterone |
AlP |
6 |
0.27 |
0.08 |
0.18 |
0.36 |
<0.0001 |
| AlP-KNO3-L |
6 |
0.29 |
0.05 |
0.24 |
0.34 |
| AlP-KNO3-H |
6 |
0.36 |
0.18 |
0.18 |
0.55 |
| AlP-CaCO3-L |
6 |
0.15 |
0.09 |
0.06 |
0.24 |
| AlP-CaCO3-H |
6 |
0.17 |
0.33 |
0.14 |
0.21 |
| AlP-NaBr-L |
6 |
0.81 |
0.31 |
0.48 |
1.13 |
| AlP-NaBr-H |
6 |
0.56 |
0.17 |
0.39 |
0.74 |
| Control |
6 |
0.53 |
0.30 |
0.50 |
0.56 |
| Progesterone |
AlP |
6 |
26.57 |
8.19 |
17.97 |
35.16 |
<0.0001 |
| AlP-KNO3-L |
6 |
20.97 |
5.25 |
15.46 |
26.47 |
| AlP-KNO3-H |
6 |
5.00 |
1.01 |
3.94 |
6.06 |
| AlP-CaCO3-L |
6 |
13.10 |
1.42 |
11.61 |
14.59 |
| AlP-CaCO3-H |
6 |
52.33 |
1.88 |
50.36 |
54.31 |
| AlP-NaBr-L |
6 |
45.00 |
1.45 |
43.48 |
46.52 |
| AlP-NaBr-H |
6 |
34.20 |
1.42 |
32.71 |
35.69 |
| Control |
6 |
48.20 |
1.59 |
46.53 |
49.78 |
| KNO3-H |
6 |
38.50 |
15.35 |
22.40 |
54.60 |
| CaCO3-H |
6 |
2.52 |
0.28 |
2.22 |
2.81 |
| NaBr-H |
6 |
31.60 |
22.63 |
7.86 |
55.34 |
| Estradiol |
AlP |
6 |
16.20 |
0.32 |
15.87 |
16.53 |
<0.0001 |
| AlP-KNO3-L |
6 |
20.70 |
1.06 |
19.58 |
21.82 |
| AlP-KNO3-H |
6 |
23.47 |
5.16 |
18.05 |
28.89 |
| AlP-CaCO3-L |
6 |
17.60 |
0.56 |
17.01 |
18.19 |
| AlP-CaCO3-H |
6 |
13.80 |
0.26 |
13.52 |
14.08 |
| AlP-NaBr-L |
6 |
13.90 |
0.43 |
13.45 |
14.36 |
| AlP-NaBr-H |
6 |
7.40 |
1.52 |
5.81 |
8.99 |
| Control |
6 |
8.63 |
2.64 |
5.86 |
11.41 |
| KNO3-H |
6 |
16.52 |
1.64 |
14.80 |
18.24 |
| CaCO3-H |
6 |
16.90 |
0.93 |
15.92 |
17.88 |
| NaBr-H |
6 |
10.25 |
1.54 |
8.64 |
11.86 |
The FSH hormone in rats receiving AlP-CaCO3-L were significantly higher compared to that in the AlP (P<0.0001), AlP-KNO3-L (P<0.0001), AlP-KNO3-H (P<0.0001), AlP-CaCO3-H (P<0.0001), AlP-NaBr-L (P<0.0001), AlP-NaBr-H (P<0.0001), control (P<0.0001), KNO3-H (P<0.0001), CaCO3-H (P<0.0001) and NaBr-H (P<0.0001) groups.
In addition, the LH hormone in rats receiving AlP-NaBr-L was substantially higher compared to that in the AlP (P=0.001), AlP-KNO3-L (P=0.001), AlP-NaBr-H (P=0.003), control (P=0.002), KNO3-H (P=0.021) and CaCO3-H (P=0.039) groups. It was also demonstrated that the rats receiving NaBr-H had a higher level of LH hormone compared to its level in the AlP (P<0.0001), AlP-KNO3-L (P<0.0001), AlP-CaCO3-H (P=0.040), AlP-NaBr-H (P<0.0001), control (P<0.0001), KNO3-H (P=0.004) and CaCO3-H (P=0.008) groups.
According to the obtained results, the level of prolactin hormone in the rats receiving AlP- KNO3-L was significantly higher than that in the rats receiving AlP (P=0.008), AlP-KNO3-H (P=0.017), AlP- CaCO3-L (P=0.050), AlP-NaBr-H (P=0.050) and KNO3-H (P=0.006). In addition, the rats in the control group have a higher level of prolactin hormone in comparison with its level in the AlP (P=0.003), AlP-KNO3-H (P=0.007), AlP-CaCO3-L (P=0.021), AlP-NaBr-L (P=0.043), AlP-NaBr-H (P=0.021) and KNO3-H (P=0.002) groups.
Regarding progesterone hormone, the results indicated that this hormone was significantly lower in the AlP-KNO3-H group compared to that in the AlP (P=0.004), AlP-CaCO3-H (P<0.0001), AlP-NaBr-L (P<0.0001), AlP-NaBr-H (P<0.0001), control (P<0.0001), KNO3-H (P<0.0001) and NaBr-H (P<0.0001). Meanwhile, progesterone in rats receiving AlP-CaCO3-L was lower than that in the AlP-CaCO3-H (P < 0.0001), AlP-NaBr-L (P < 0.0001), AlP-NaBr-H (P=0.005), control (P<0.0001), KNO3-H (P<0.0001) and NaBr-H (P=0.024). Moreover, progesterone in the rats receiving AlP-CaCO3-H was significantly higher compared to that in the groups receiving AlP (P<0.0001), AlP-KNO3-L (P<0.0001), AlP-NaBr-H (P<0.0001), CaCO3-H (P<0.0001) and NaBr-H (P=0.006). This hormone was significantly higher in the AlP-NaBr-L receiving group than in the AlP (P=0.025), AlP-KNO3-L (P=0.001), and CaCO3-H (P<0.0001) groups. It was also significantly higher than AlP (P=0.004) and AlP-KNO3-L (P<0.0001) in control rats. Finally, it was determined that the rats receiving CaCO3-H had lower progesterone levels than that in the AlP (P=0.001), AlP-KNO3-L (P=0.024), AlP-CaCO3-H (P<0.0001), AlP-NaBr-L (P<0.0001), AlP-NaBr-H (P<0.0001), control (P<0.0001), KNO3-H (P<0.0001) and NaBr-H (P<0.0001).
The results related to estradiol hormone showed that rats poisoned with AlP had a lower level of estradiol than AlP-KNO3-L (P=0.011), AlP-KNO3-H (P<0.0001), AlP-NaBr-H (P<0.0001), control (P<0.0001) and NaBr-H (P<0.0001). Rats receiving ALP-KNO3-L had a higher level of estradiol in comparison with AlP-CaCO3-H (P<0.0001), AlP-NaBr-L (P<0.0001), AlP-NaBr-H (P<0.0001), control (P<0.0001), KNO3-H (P=0.023) and NaBr-H (P<0.0001). It was also indicated that rats receiving AlP-KNO3-H had higher estradiol levels than those in other groups except rats receiving AlP-KNO3-L (P<0.0001). In the group receiving AlP-CaCO3-L, the amount of estradiol was higher compared to that in the rats receiving AlP-NaBr-H (P<0.0001), control (P<0.0001) and NaBr-H (P<0.0001). Rats receiving AlP-CaCO3-H had a higher level of estradiol in comparison with that in the AlP-NaBr-H (P<0.0001) and control (P=0.002). Rats receiving AlP-NaBr-L had a higher level of estradiol in comparison with that in the AlP-NaBr-H (P<0.0001) and control (P=0.001). Rats receiving KNO3-H have a higher level of estradiol in comparison with its level in rats receiving AlP-NaBr-H (P<0.0001), control (P<0.0001), and NaBr-H (P<0.0001). Finally, it was determined that the group receiving CaCO3-H had a higher level of estradiol in comparison with that in the rats receiving AlP-NaBr-H (P<0.0001), normal control (P<0.0001), and NaBr-H. (P<0.0001).
Discussion
The present study aimed to examine the impact of novel antidotes on AlP poisoning in digestive tissues, ovaries, the uterus, and several reproduction hormones in a rat animal model. These antidotes included NaBr, CaCO3, and KNO3 compounds. The findings of our investigation demonstrated that the tissues of the esophagus, stomach, pancreas, uterus, and ovary exhibited no substantial and noteworthy modifications in histological tests. The levels of the LH, progesterone, and estradiol hormones were significantly negatively associated with AlP concentration, whereas hormonal investigations revealed significant variations across groups. Additionally, the FSH, LH, and prolactin hormones in the AlP- CaCO3, NaBr, and control groups, respectively, had higher levels than those in the other groups. In contrast to other groups, progesterone hormone levels significantly decreased in the AlP-KNO3 and CaCO3 groups. Regarding the hormone estradiol, the results indicated that the level of estradiol was relatively higher in rats receiving AlP and AlP-KNO3.
As mentioned earlier, FSH levels were greater in the AlP-CaCO3 group than in the other groups. An increase in FSH is a sign of declining ovarian reserve. A decline in ovarian reserve is linked to reduced follicles or eggs, which are frequently of low quality. Statistics show that women with high FSH levels have a poor likelihood of conceiving a child. According to this work, additional substances (e.g., KNO3 and NaBr) had no discernible impact on the FSH hormone levels compared to the control group. Loeber et al., using immunocytochemistry and radioimmunoassay, aimed to investigate how NaBr affected rat endocrine parameters. The findings of their investigation demonstrated that NaBr directly interferes with thyroid, testicular, and adrenal activities, including the activation of the FSH hormone, at least at high levels. It appears that low doses of NaBr do not have a stimulating effect on FSH, which supports our study because the highest dose we used in this study was 370 mg/kg, which did not have a stimulating effect on FSH. In this study, 0, 20, 75, 300, 1200, and 19,200 mg/kg doses were used, and the effect of NaBr was stimulating only at high doses [38]. In a study on the effects of NaBr on people, with a focus on the endocrine system, Sangster et al. found that the FSH hormone did not alter considerably because of the injection of NaBr [39]. However, we reported that KNO3 did not alter FSH, and no research has been conducted on this topic. Our investigation into the FSH hormone generally has shown that AlP has no impact on the amount of this hormone.
The LH hormone was also examined in this study, and the findings revealed that AlP had no discernible impact on this hormone. LH aids in the regulation of the menstrual cycle in women. Additionally, ovulation—the release of the egg from the ovary—is brought on by it. Just prior to ovulation, LH levels rise quickly. Only NaBr was able to considerably increase the level of this hormone in our investigation; there was no discernible difference in hormone levels between the other groups. As a result, despite an inverse association between AlP concentration and LH hormone, it can be said that AlP does not significantly affect LH hormone compared to the control group because no additional research in this field has been carried out. Therefore, it is suggested that more studies be conducted in this field.
Prolactin was another hormone examined in this study, and the results demonstrated a significant decrease in this hormone in rats receiving AlP; therefore, it can be concluded that this hormone is affected by AlP poisoning. Interestingly, the prolactin hormone in rats receiving AlP treated with KNO3 was not different from the control group, and it indicates that KNO3 can neutralize the effect of AlP toxicity on the level of prolactin hormone. Other evaluated compounds did not show such an effect, and the amount of prolamin in rats receiving AlP after treatment with NaBr and CaCO3 significantly decreased compared to the control group. A decrease in the amount of secreted prolactin can lead to insufficient milk production after childbirth. Most people with low prolactin levels do not have any specific medical problems, although early evidence suggests they may have reduced immune responses to some infections. So far, no study has been conducted regarding the effect of AlP or KNO3 on the level of prolactin hormone. Therefore, it can be acknowledged that in this study, for the first time, we indicated the reducing effect of AlP on the prolactin hormone and the antidote effect of KNO3 on the toxicity of AlP regarding this hormone.
Another hormone whose level changed significantly after treatment with AlP in our study was estradiol hormone, which significantly increased after treatment of rats with AlP compared to the control group. Both males and females produce estradiol, the most common type of estrogen in women during their reproductive years. Significant levels of estradiol may lead to acne, decreased libido, osteoporosis, and depression. In addition, its high levels can increase the risk of uterine and breast cancer. Therefore, it can be concluded that the effect of AlP on estradiol can lead to many diseases. On the other hand, based on the findings of this study, the level of estradiol in rats poisoned with AlP and treated with KNO3 and NaBr was not significantly different from the control group. Therefore, the results of our study indicate the antidote effect of these two substances on the toxicity caused by AlP on the level of estradiol hormone.
Conclusions
Results of our study showed that AlP had no adverse effect on the tissues of the esophagus, stomach, pancreas, uterus, or ovaries of the studied rats. On the other hand, the levels of LH, estradiol, and prolactin hormones showed an inverse relationship with AlP. According to the findings, AlP significantly affected the levels of estradiol and prolactin hormones compared to the control group in such a way that it decreased and increased prolactin and estradiol, respectively. Among the investigated antidotes, KNO3 was seen as an effective antidote against AlP toxicity in prolactin and estradiol, and NaBr effectively reduced the effect of AlP toxicity.
Data Access and Responsibility
As the principal investigator and corresponding author of the study, Ali Ostadi has full access to all the study’s data and bears responsibility for its accuracy and integrity.
Ethical Considerations
Compliance with ethical guidelines: This study was performed in compliance with the relevant laws and institutional guidelines and has been approved by the ethics committee of Tabriz Medical School with the ethics code IR.TBZMED.VCR.REC.1399.353. All the ethical principles of working with animals were considered by the researchers.
Authors' Contributions
All authors were involved in Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Project Administration, Resources, Software, Supervision, Validation, Visualization, Original Draft, as well as Review & Editing.
Acknowledgement
This work has been supported by the Deputy of Research and Technology, Tabriz University of Medical Sciences, Iran (grant number 67142, Ethical Code: IR.TBZMED.VCR.REC.1399.353). The results presented in this study were extracted from Negin Soltanieh's MD thesis.
Conflict of Interests
There are no conflicts of interest to declare.
Funding
This study has been supported by Tabriz University of Medical Sciences, Deputy of Research and Technology (grant number 67142).
References
- Chopra JS, Kalra OP, Malik VS, Sharma R, Chandna A. Aluminium phosphide poisoning: a prospective study of 16 cases in one year. Postgraduate medical journal. 1986;62(734):1113-5. [DOI: 10.1136/pgmj.62.734.1113] [PMID: 3658848]
- Mehrpour O, Singh S. Rice tablet poisoning: a major concern in Iranian population. Human & experimental toxicology. 2010;29(8):701-2. [DOI: 10.1177/0960327109359643] [PMID: 20097728]
- Wahab A, Rabbani MU, Wahab S, Khan RA. Spontaneous self-ignition in a case of acute aluminium phosphide poisoning. The American journal of emergency medicine. 2009;27(6):752.e5-6. [DOI: 10.1016/j.ajem.2008.09.045] [PMID: 19751640]
- Mehrpour O, Alfred S, Shadnia S, Keyler DE, Soltaninejad K, Chalaki N, et al. Hyperglycemia in acute aluminum phosphide poisoning as a potential prognostic factor. Human & experimental toxicology. 2008;27(7):591-5. [DOI: 10.1177/0960327108096382] [PMID: 18829736]
- Nosrati A, Karami M, Esmaeilnia M. Aluminum Phosphide Poisoning: A Case Series in North Iran. Asia Pacific Journal of Medical Toxicology. 2013;2(3):111-3. [DOI: 10.22038/apjmt.2013.1674]
- Anbalagan LC, Arora N, Pannu AK. Management of Acute Aluminum Phosphide Poisoning: Has Anything Changed?. Drug metabolism letters. 2021;14(2):106-16.[ DOI: 10.2174/1872312814666210813115625] [PMID: 34818996]
- Mehrpour O, Jafarzadeh M, Abdollahi M. A systematic review of aluminium phosphide poisoning. Arhiv za higijenu rada i toksikologiju. 2012;63(1):61-73. [DOI: 10.2478/10004-1254-63-2012-2182] [PMID: 22450207]
- Aggarwal P, Handa R, Wig N, Biswas A, Saxena R, Wali JP. Intravascular hemolysis in aluminium phosphide poisoning. The American journal of emergency medicine. 1999;17(5):488-9. [DOI: 10.1016/s0735-6757(99)90255-3] [PMID: 10496516]
- Chugh SN, Arora V, Sharma A, Chugh K. Free radical scavengers & lipid peroxidation in acute aluminium phosphide poisoning. The Indian journal of medical research. 1996;104:190-3. [PMID: 8840658]
- Jaiswal S, Verma RK, Tewari N. Aluminum phosphide poisoning: effect of correction of severe metabolic acidosis on patient outcome. Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2009;13(1):21-4. [DOI: 10.4103/0972-5229.53111] [PMID: 19881175]
- Mehrpour O, Aghabiklooei A, Abdollahi M, Singh S. Severe hypoglycemia following acute aluminum phosphide (rice tablet) poisoning; a case report and review of the literature. Acta medica Iranica. 2012;50(8):568-71. [PMID: 23109032]
- Sudakin DL. Occupational exposure to aluminium phosphide and phosphine gas? A suspected case report and review of the literature. Human & experimental toxicology. 2005;24(1):27-33. [DOI: 10.1191/0960327105ht496oa] [PMID: 15727053]
- Moghadamnia AA. An update on toxicology of aluminum phosphide. Daru : journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2012;20(1):25. [DOI: 10.1186/2008-2231-20-25] [PMID: 23351193]
- Bagheri-Moghaddam A, Abbaspour H, Tajoddini S, Mohammadzadeh V, Moinipour A, Dadpour B. Using Intra-Aortic Balloon Pump for Management of Cardiogenic Shock Following Aluminum Phosphide Poisoning; Report of 3 Cases. Emergency (Tehran, Iran). 2018;6(1):e3. [PMID: 29503828]
- Hassanian-Moghaddam H, Zamani N, Rahimi M, Hajesmaeili M, Taherkhani M, Sadeghi R. Successful Treatment of Aluminium Phosphide Poisoning by Extracorporeal Membrane Oxygenation. Basic & clinical pharmacology & toxicology. 2016;118(3):243-6. [DOI: 10.1111/bcpt.12481] [PMID: 26335576]
- de Lange DW, Sikma MA, Meulenbelt J. Extracorporeal membrane oxygenation in the treatment of poisoned patients. Clinical toxicology (Philadelphia, Pa). 2013;51(5):385-93. [DOI: 10.3109/15563650.2013.800876] [PMID: 23697460]
- Mohan B, Singh B, Gupta V, Ralhan S, Gupta D, Puri S, et al. Outcome of patients supported by extracorporeal membrane oxygenation for aluminum phosphide poisoning: An observational study. Indian heart journal. 2016;68(3):295-301. [DOI: 10.1016/j.ihj.2016.03.024] [PMID: 27316480]
- Siwach SB, Singh P, Ahlawat S, Dua A, Sharma D. Serum & tissue magnesium content in patients of aluminium phosphide poisoning and critical evaluation of high dose magnesium sulphate therapy in reducing mortality. The Journal of the Association of Physicians of India. 1994;42(2):107-10. [PMID: 7860467]
- Chugh SN, Kumar P, Aggarwal HK, Sharma A, Mahajan SK, Malhotra KC. Efficacy of magnesium sulphate in aluminium phosphide poisoning--comparison of two different dose schedules. The Journal of the Association of Physicians of India. 1994;42(5):373-5. [PMID: 7829435]
- Chugh SN, Kolley T, Kakkar R, Chugh K, Sharma A. A critical evaluation of anti-peroxidant effect of intravenous magnesium in acute aluminium phosphide poisoning. Magnesium research. 1997;10(3):225-30. [PMID: 9483483]
- Karimani A, Mohammadpour AH, Zirak MR, Rezaee R, Megarbane B, Tsatsakis A, et al. Antidotes for aluminum phosphide poisoning - An update. Toxicology reports. 2018;5:1053-9. [DOI: 10.1016/j.toxrep.2018.10.009] [PMID: 30406022]
- Shadnia S, Rahimi M, Pajoumand A, Rasouli MH, Abdollahi M. Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil. Human & experimental toxicology. 2005;24(4):215-8. [DOI: 10.1191/0960327105ht513oa] [PMID: 15957538]
- Agrawal VK, Bansal A, Singh RK, Kumawat BL, Mahajan P. Aluminum phosphide poisoning: Possible role of supportive measures in the absence of specific antidote. Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2015;19(2):109-12. [DOI: 10.4103/0972-5229.151019] [PMID: 25722553]
- Tehrani H, Halvaie Z, Shadnia S, Soltaninejad K, Abdollahi M. Protective effects of N-acetylcysteine on aluminum phosphide-induced oxidative stress in acute human poisoning. Clinical toxicology (Philadelphia, Pa). 2013;51(1):23-8. [DOI: 10.3109/15563650.2012.743029] [PMID: 23148565]
- Manoochehri A, Ahangar RM, Bigvand P, Nakhaee S, Mehrpour O. A Case of Successful Treatment of Heart Failure Due to Simultaneous Poisoning with Aluminum Phosphide and Zinc Phosphide. Iranian Red Crescent Medical Journal. 2018. [DOI: 10.5812/ircmj.57123]
- Bhalla A, Jyothinath P, Singh S. Antioxidant Therapy in Patients with Severe Aluminum Phosphide Poisoning: A Pilot Study. Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2017;21(12):836-40. [DOI: 10.4103/0972-5229.220744] [PMID: 29307964]
- Agarwal A, Robo R, Jain N, Gutch M, Consil S, Kumar S. Oxidative stress determined through the levels of antioxidant enzymes and the effect of N-acetylcysteine in aluminum phosphide poisoning. Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2014;18(10):666-71. [DOI: 10.4103/0972-5229.142176] [PMID: 25316977]
- Moghadam Nia A, Firooz Jahi A, Javadian S, Dibavand N. Aluminium phosphide poisoning in mice and the procedure for its managements . Journal of Babol University of Medical Sciences. 2000;2(4):25-33. [Link]
- Oghabian Z, Mehrpour O. Treatment of Aluminium Phosphide Poisoning with a Combination of Intravenous Glucagon, Digoxin and Antioxidant Agents. Sultan Qaboos University medical journal. 2016;16(3):e352-5. [DOI: 10.18295/squmj.2016.16.03.015] [PMID: 27606117]
- Halvaei Z, Tehrani H, Soltaninejad K, Abdollahi M, Shadnia S. Vitamin E as a novel therapy in the treatment of acute aluminum phosphide poisoning. Turkish journal of medical sciences. 2017;47(3):795-800. [DOI: 10.3906/sag-1512-6] [PMID: 28618724]
- Abdolghaffari AH, Baghaei A, Solgi R, Gooshe M, Baeeri M, Navaei-Nigjeh M, et al. Molecular and biochemical evidences on the protective effects of triiodothyronine against phosphine-induced cardiac and mitochondrial toxicity. Life sciences. 2015;139:30-9.[ DOI: 10.1016/j.lfs.2015.07.026] [PMID: 26239436]
- Goharbari MH, Taghaddosinejad F, Arefi M, Sharifzadeh M, Mojtahedzadeh M, Nikfar S, et al. Therapeutic effects of oral liothyronine on aluminum phosphide poisoning as an adjuvant therapy: A clinical trial. Human & experimental toxicology. 2018;37(2):107-17. [DOI: 10.1177/0960327117694074] [PMID: 29233028]
- Türkez H, Toğar B. Aluminium phosphide-induced genetic and oxidative damages in vitro: attenuation by Laurus nobilis L. leaf extract. Indian journal of pharmacology. 2013;45(1):71-5. [DOI: 10.4103/0253-7613.106439] [PMID: 23543905]
- Soltani M, Shetab-Boushehri SF, Shetab-Boushehri SV. Chemical Reaction between Boric Acid and Phosphine Indicates Boric Acid as an Antidote for Aluminium Phosphide Poisoning. Sultan Qaboos University medical journal. 2016;16(3):e303-9. [DOI: 10.18295/squmj.2016.16.03.007] [PMID: 27606109]
- Baghaei A, Solgi R, Jafari A, Abdolghaffari AH, Golaghaei A, Asghari MH, et al. Molecular and biochemical evidence on the protection of cardiomyocytes from phosphine-induced oxidative stress, mitochondrial dysfunction and apoptosis by acetyl-L-carnitine. Environmental toxicology and pharmacology. 2016;42:30-7. [DOI: 10.1016/j.etap.2015.12.019] [PMID: 26773361]
- Salimi A, Paeezi M, Yousefsani BS, Shadnia S, Hassanian-Moghaddam H, Zamani N, et al. Inhibition of glucose-6-phosphate dehydrogenase protects hepatocytes from aluminum phosphide-induced toxicity. Pesticide biochemistry and physiology. 2017;143:141-6. [DOI: 10.1016/j.pestbp.2017.08.005] [PMID: 29183584]
- Klopfleisch R. Multiparametric and semiquantitative scoring systems for the evaluation of mouse model histopathology--a systematic review. BMC veterinary research. 2013;9:123. [DOI: 10.1186/1746-6148-9-123] [PMID: 23800279]
- Loeber JG, Franken MA, van Leeuwen FX. Effect of sodium bromide on endocrine parameters in the rat as studied by immunocytochemistry and radioimmunoassay. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1983;21(4):391-404. [DOI: 10.1016/0278-6915(83)90093-5] [PMID: 6352433]
- Sangster B, Krajnc EI, Loeber JG, Rauws AG, van Logten MJ. Study of sodium bromide in human volunteers, with special emphasis on the endocrine system. Human toxicology. 1982;1(4):393-402. [DOI: 10.1177/096032718200100405] [PMID: 7173924]










































 , Ali Ostadi *2
, Ali Ostadi *2 

 , Mehran Mesgari-Abbasi1
, Mehran Mesgari-Abbasi1 

 , Monireh Khordadmehr3
, Monireh Khordadmehr3 

 , Azin Behrouzi-Kahlan1
, Azin Behrouzi-Kahlan1 

 , Ali Banagozar Mohammadi1
, Ali Banagozar Mohammadi1 

 , Alireza Ghaffari1
, Alireza Ghaffari1 

 , Ilghar Najafirad1
, Ilghar Najafirad1 







