Ethics code: AERC/2022/090


Arinze K, Amole O, Dada A, Ojewale A, Emiogun E, Yemitan O. Antihepatotoxic Effects of Hydro ethanol Leaf and Aqueous Unripe Whole Fruit Extracts of Carica papaya on Paracetamol-induced Liver Injury in Rats. IJT 2025; 19 (3) :146-152
URL:
http://ijt.arakmu.ac.ir/article-1-1455-en.html
1- Department of Pharmacology, Therapeutics and Toxicology, Lagos State University College of Medicine, Ikeja, Lagos State, Nigeria.
2- Department of Chemical Pathology, Lagos State University College of Medicine, Ikeja, Lagos State, Nigeria.
3- Department of Anatomy, Lagos State University College of Medicine, Ikeja, Lagos State, Nigeria.
4- Department of Pathology and Forensic Medicine, Lagos State University College of Medicine, Ikeja, Lagos State, Nigeria.
5- Department of Pharmacology, Therapeutics and Toxicology, Lagos State University College of Medicine, Ikeja, Lagos State, Nigeria. , omoniyi.yemitan@lasucom.edu.ng
Full-Text [PDF 821 kb]
(155 Downloads)
|
Abstract (HTML) (1080 Views)
Full-Text: (143 Views)
Introduction
Traditional medicine, particularly herbal therapy, has remained pivotal in the health system since the origin of humankind. Reports show that it is a prevalent alternative therapy in developing countries, especially African countries, when compared to orthodox therapy, with about 80% of Africans relying on these alternative therapies regardless of modern medical practices [1]. Among these medicinal plants, Carica papaya (family Caricaceae), commonly known as pawpaw, is popularly used. Although native to the Americas, it is extensively cultivated in tropical and sub-tropical regions worldwide [2]. Carica papaya )C. papaya) has been employed as a folk medicine in many countries because of its therapeutic features, such as antioxidative, antimalarial, antihypertensive, antidiabetic, and antimicrobial properties [3-7]. Studies reveal that these therapeutic properties are attributed to the abundance of chemicals, such as papain, chymopapain, carotenoids, monoterpenoids, saponins, cardenolides, glucosinolates, potassium, calcium, iron, phosphorus, zinc and copper in the fruit extracts, whereas the leaves contain papain, chymopapain, cystain, ascorbic acid, alkaloids, flavonoids, cyanogenic glucosides and minerals [8-10].
Paracetamol (PCM) is a widely used first-choice analgesic for mild to moderate acute pain in adults, especially in those with underlying health conditions like ulcers, pregnancy, and lactation, where non-steroidal anti-inflammatory drugs (NSAIDs) may pose risks [11].
The liver is the main organ responsible for the metabolism and detoxification of most xenobiotics, including commonly used analgesics like PCM and herbal products [12,13]. It is a prime target for chemical-induced injury, particularly drug-induced liver injury (DILI), as the hepatotoxic metabolite of the drug binds to basic cellular components and adversely induces the majority of liver lesions, particularly N-acetyl-p-benzoquinone imine (NAPQI), an active and dose-dependent hepatotoxic metabolite of PCM [14] The DILI continues to be the leading reason for post-marketing regulatory decisions, including drug discontinuation, as over a thousand drugs in the updated pharmaceuticals pharmacopeia issue, accounting for approximately 90% of all cases of withdrawn over-the-counter drugs and herbal supplements from clinical trials and the market, as reported by scientists [15] In addition, orthodox therapeutic options for liver diseases are very limited, resulting in great demand for traditional and alternative therapy, particularly herbal plants like C. papaya [16,17]
Despite the individual effectiveness of the parts of C. papaya, there is a dearth of scientific knowledge on the comparison of the acute antihepatotoxicity models between the leaf and fruit extracts of C. papaya, particularly against PCM-induced hepatotoxicity in rats. Therefore, the present study compared the hepatoprotective activity of hydroethanol leaf and aqueous fruit extracts of C. papaya against PCM-induced hepatotoxicity in rats.
Materials and Methods
Plant Material
Freshly harvested leaves and unripe fruits of C. papaya were collected from the Lagos State University College of Medicine garden in Ikeja, Lagos State (Nigeria) with coordinates 6.58845⁰ N and 3.34161⁰ E in January 2023. Sequel to the collection, both plant samples were identified using the PictureThisÒ Apple Store App and authenticated by Dr. George Ndoza of the Department of Botany, Faculty of Science at the University of Lagos, Lagos, Nigeria, where a voucher specimen was deposited and given the identification number: LUH 9852 on February 6, 2023.
Preparation of Extracts
The procedure followed a protocol previously conducted by Awodele et al [5]. Leaves of C. papaya were washed under running tap water, oven-dried at 40°C, blended to a coarse powder, and weighed. Then, 100 g of the 400 g of leaves were macerated in 4.0 L of hydroethanol mixture (50:50) at 70°C for 4 h using a heating mantle until a thick concentrated solution was formed, which was allowed to cool and filtered using filter paper. The filtrate was placed inside the water bath at 75°C for 4 h to attain the dried extract (yield: 5 %), which was then refrigerated at 4°C from which fresh preparations were made throughout the study.
For the unripe fruit, 300 g of whole fruit of C. papaya was washed, peeled, cut into small pieces, and soaked in 100 mL of distilled water for 7 days. The solution was systematically filtered using a clean, dry muslin cloth and filter paper. The filtrate was refrigerated at 4°C (yield: 9 %) and used throughout the study.
Chemicals and Kits
PCM was purchased from the Lagos State University Fee-paying Pharmacy, Ikeja, Lagos State, Nigeria. Assay kits for biochemical evaluation of serum liver biomarkers, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and uric acid, were all purchased from Fortress Diagnostics, United Kingdom. All other analytical-grade reagents were obtained from the Department of Chemical Pathology, Lagos State University College of Medicine, Ikeja, Lagos State, Nigeria.
Experimental Animals
Male adult albino rats (weighing 180-200 g) procured from the Animal House, Lagos State College of Medicine, Nigeria, were used. The animals were housed at standard environmental conditions with an optimal temperature of 22±2°C, a 12-h light-dark cycle, while being fed standard animal feed and tap water ad libitum for two weeks before the experimentation. All animal procedures deployed in this study fully adhered to the ethical procedures and policies with due approval from the Animal Ethical Research Committee (AERC) of Lagos State University (Reference No. AERC/2022/090).
Acute Toxicity Study
The oral median lethal dose (LD50) of C. papaya in rats was determined according to the procedures outlined by the Organization for Economic Co-operation and Development (OECD) [18] by weighing the body weight of the rats after a 24-h fasting period. In the first phase, six rats were randomly allotted into three groups (n=2). They were administered 10 mg/kg, 100 mg/kg, and 1000 mg/kg of HECL and AUCF, respectively and observed for signs of debility and mortality within 24 h. Following the outcome of the first phase, four rats were divided into two groups (n=2), which were administered 2000 mg/kg and 4000 mg/kg of the HECL and AUCF, respectively and then closely monitored for any evidence of toxicity and death in 24 h during the second phase. The median lethal dose was 5000 mg/kg of body weight for each C. papaya extract. Then, 200 mg/kg and 400 mg/kg were the selected doses for HECL and AUCF throughout this experiment.
Experimental Design
PCM-induced Hepatotoxicity
A total of 30 rats were randomly divided into six treatment groups of five animals each. Group 1 (normal) received 1 mL/kg of normal saline (NS) orally at intervals of 2, 6, and 10 h. Group II (control) received PCM at 600 mg/kg of PCM solution containing PCM dissolved in NS, followed by 3 ml/kg of NS at 2, 6, and 10 h. Groups III and IV received oral administration of 600 mg/kg of PCM, followed by post-administration of HECL at 200 mg/kg and 400 mg/kg doses, respectively, at 2, 6, and 10 h intervals. Groups V and VI received oral administration of 600 mg/kg of PCM, followed by post-administration of AUCF at doses of 200 mg/kg and 400 mg/kg, respectively, at intervals of 2, 6, and 10 h.
All the treatments were administered orally using an oral cannula, and the doses of HECL or AUCF were selected based on pre-tests.
Collection of Samples
All animals were refrained from feeding for 24 h after treatment and were anaesthetized by ether inhalation. Blood samples were collected from the heart via a cardiac puncture and allowed to clot for 30 min. The serum was separated by centrifugation at 2500 rpm for 15 min and used for biochemical assessment. The liver was excised, washed with saline, blotted dry, divided into samples, frozen quickly, and stored at 80°C. The fixed tissues were processed routinely, embedded in paraffin, sectioned to 3–5 mm thickness, deparaffinized and rehydrated using standard techniques. Sections were taken and stained with hematoxylin and eosin (H&E).
Biochemical Assessment of Serum Liver Enzymes
The effect of treatment on serum ALT, AST and uric acid concentration was determined using the Fortress Diagnostics Standard Kits (United Kingdom).
Effect of Treatment with PCM on the Serum ALT
The procedure for the assessment of ALT complied with a previous study and was modified by the Fortress Diagnostics Standard Operating Procedure [19]. The preparation containing 100 uL of the substrate dissolved in 500 uL of the buffer (containing phosphate, L-alanine and α-oxoglutarate) followed by 500 uL of dinitrophenylhydrazine (a dye reagent) dissolved in 1.5 ml of diluted sodium hydroxide preparation (1 part to 19 parts of deionized water) and the absorbance was read using a spectrophotometer against the standard wavelength of 550 nm.
Effect of Treatment with PCM on the Serum AST
The procedure for the assessment of AST was similar to that of ALT, which complied with a previous study as modified by Fortress Diagnostics Standard Operating Procedure [19]. The preparation containing 100 uL of the substrate dissolved in 500 uL of the buffer (containing phosphate, L-aspartate and α-oxoglutarate) followed by 500 uL of dinitrophenylhydrazine (dye reagent) was dissolved in 1.5 ml of diluted sodium hydroxide preparation (1 part to 19 parts of deionized water) and the absorbance was read using a spectrophotometer against the standard wavelength of 550 nm.
Effect of Treatment with PCM on the Levels of Uric Acid
Uric acid was accessed according to the method described in a study by Fawcett and Scott [20]. 20 µL of the sample (containing serum) was added to uric acid reagent preparation consisting of 50 mmol/L of hepes buffer, 4.0 mmol/L of 3,5-dichloro-2-hydroxybenzenesulfonic acid (DCHBS), 1 KU/L of peroxidase and 0.2 KU/L of uricase. Then, it was mixed thoroughly and incubated for 5 min at 37°C. The absorbance of the solution was read with the aid of a spectrophotometer with a standard wavelength of 546 nm.
Histopathological Assessment
Animals from each study were sacrificed, and the liver of each animal was excised immediately. A portion of the liver was fixed in a 10% neutral buffered formalin solution for 24 h. Afterward, they were processed routinely, embedded in paraffin, and cut into 5 μm-thick sections using a microtome. Then, they were deparaffinized, rehydrated using standard techniques, and stained with H&E before the photomicroscopy at a magnification of X 40 for morphological pathology, including necrosis, steatosis, and fatty change of hepatic cells and hepatocyte damage. The rest of the livers were rinsed with saline, blotted dry, divided into samples, frozen quickly, and stored at 80°C (Saeed and Sabir, 2002).
Statistical analysis
Results were expressed as mean±SEM. Graphpad Prism (version 8.0) analytical models, particularly the one-way analysis of variance (ANOVA) followed by multiple comparisons using Tukey’s post-hoc test, were used for all the statistical analyses and graphical representations of the comparison of the biochemical parameters between the groups, with P<0.05 considered statistically significant.
Results
Acute Toxicity Studies
No mortality was observed up to a dose level of 5000 mg/kg body weight. When compared with the control, no observable changes were observed in their morphological, behavioral, feeding pattern or water intake during the acute toxicity test.
Effects of Leaf and Fruit Extracts of C. papaya Against PCM-induced Liver Injury on ALT Levels
PCM (600 mg/kg) produced a significant liver injury, as indicated by a significant increase in the serum concentration of ALT compared to control, HECL, and AUCF (Figure 1). However, post-treatment of 200 mg/kg and 400 mg/kg HECL and AUCF caused significant decreases in ALT levels (P<0.05); however, at 400 mg/kg HECL and AUCF, a significant reduction of ALT levels was produced when compared to 200 mg/kg and 400 mg/kg HECL and AUCF (Figure 1).
In addition, 400 mg/kg of HECL produced an observable decrease in ALT levels compared to 200 mg/kg of HECL. Similarly, 400 mg/kg of AUCF produced a less observable decrease in ALT levels when compared with 200 mg/kg of AUCF.
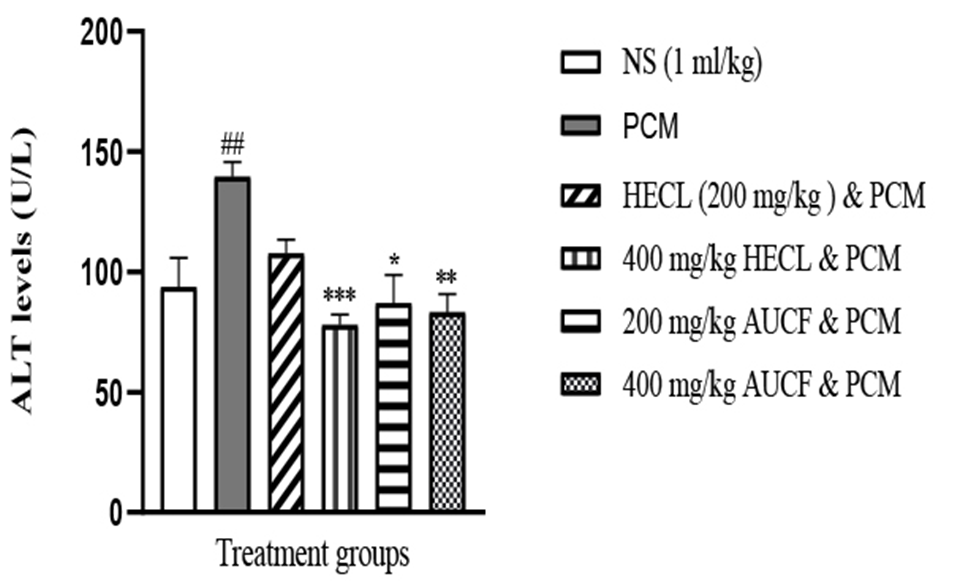 Figure 1. Showing ALT levels across all groups (n = 5). ##p < 0.01 versus N.S, *p < 0.05, **p < 0.01, ***p < 0.001 versus PCM. NS: Normal Saline, PCM: Paracetamol, HECL: Hydroethanol C. papaya leaf, AUCF: Aqueous unripe C. papaya fruit, ALT: Alanine aminotransferase
Effects of Leaf and Fruit Extracts of C. papaya Against PCM-induced Liver Injury on AST Levels
PCM (600 mg/kg) produced a significant liver injury, as indicated by a significant increase in the serum concentration of AST compared to the control, HECL, and AUCF (Figure 2). However, post-treatment of 200 mg/kg and 400 mg/kg HECL and AUCF caused significant decreases in ALT levels (P<0.05); however, 400 mg/kg HECL and AUCF produced a significant reduction of ALT levels when compared with 200 mg/kg HECL and AUCF (Figure 2).
Furthermore, 400 mg/kg of HECL produced significantly reduced AST levels compared to 200 mg/kg of HECL. Similarly, 400 mg/kg of AUCF produced a significant reduction in AST levels compared to 200 mg/kg of AUCF.
Figure 1. Showing ALT levels across all groups (n = 5). ##p < 0.01 versus N.S, *p < 0.05, **p < 0.01, ***p < 0.001 versus PCM. NS: Normal Saline, PCM: Paracetamol, HECL: Hydroethanol C. papaya leaf, AUCF: Aqueous unripe C. papaya fruit, ALT: Alanine aminotransferase
Effects of Leaf and Fruit Extracts of C. papaya Against PCM-induced Liver Injury on AST Levels
PCM (600 mg/kg) produced a significant liver injury, as indicated by a significant increase in the serum concentration of AST compared to the control, HECL, and AUCF (Figure 2). However, post-treatment of 200 mg/kg and 400 mg/kg HECL and AUCF caused significant decreases in ALT levels (P<0.05); however, 400 mg/kg HECL and AUCF produced a significant reduction of ALT levels when compared with 200 mg/kg HECL and AUCF (Figure 2).
Furthermore, 400 mg/kg of HECL produced significantly reduced AST levels compared to 200 mg/kg of HECL. Similarly, 400 mg/kg of AUCF produced a significant reduction in AST levels compared to 200 mg/kg of AUCF.
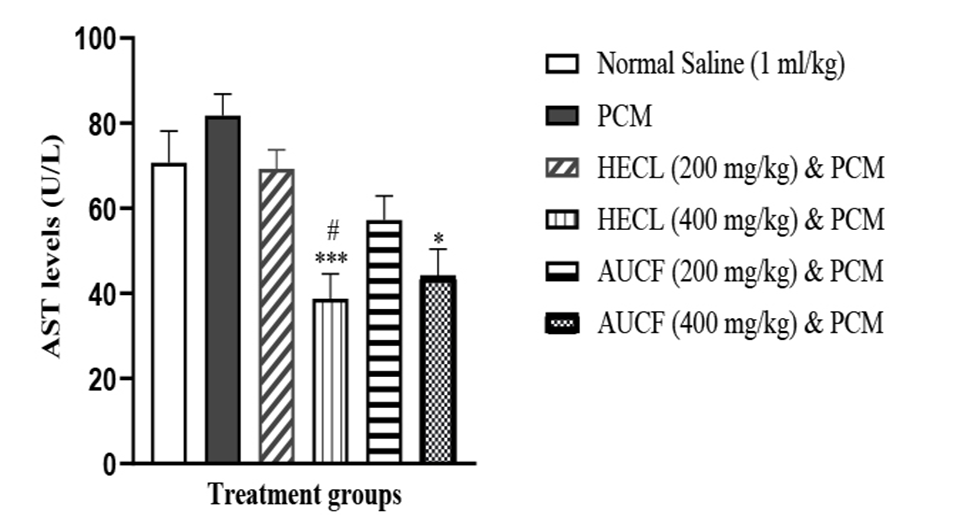 Figure 2. Showing AST levels across all groups (n = 5). #p < 0.05 versus N.S, *p < 0.05, ***p < 0.001 versus PCM. NS: Normal Saline, PCM: Paracetamol, HECL: Hydroethanol C. papaya leaf, AUCF: Aqueous unripe C. papaya fruit, AST: Aspartate aminotransferase
Effects of Leaf and Fruit Extracts of C. papaya Against PCM-induced Liver Injury on Uric Acid Levels
PCM (600 mg/kg) produced a significant liver injury, as indicated by a significant increase in the serum concentration of uric acid compared with control, HECL, and AUCF (Figure 3). However, post-treatment of 200 mg/kg and 400 mg/kg of HECL and AUCF caused significant decreases in uric acid levels (P<0.01); however, 400 mg/kg HECL and AUCF produced a significant reduction of uric acid levels when compared with 200 mg/kg HECL and AUCF (Figure 3).
Moreover, 400 mg/kg of HECL produced an observable reduction in uric acid compared to 200 mg/kg of HECL. Similarly, 400 mg/kg of AUCF produced an observable reduction in uric acid compared to 200 mg/kg of AUCF.
Figure 2. Showing AST levels across all groups (n = 5). #p < 0.05 versus N.S, *p < 0.05, ***p < 0.001 versus PCM. NS: Normal Saline, PCM: Paracetamol, HECL: Hydroethanol C. papaya leaf, AUCF: Aqueous unripe C. papaya fruit, AST: Aspartate aminotransferase
Effects of Leaf and Fruit Extracts of C. papaya Against PCM-induced Liver Injury on Uric Acid Levels
PCM (600 mg/kg) produced a significant liver injury, as indicated by a significant increase in the serum concentration of uric acid compared with control, HECL, and AUCF (Figure 3). However, post-treatment of 200 mg/kg and 400 mg/kg of HECL and AUCF caused significant decreases in uric acid levels (P<0.01); however, 400 mg/kg HECL and AUCF produced a significant reduction of uric acid levels when compared with 200 mg/kg HECL and AUCF (Figure 3).
Moreover, 400 mg/kg of HECL produced an observable reduction in uric acid compared to 200 mg/kg of HECL. Similarly, 400 mg/kg of AUCF produced an observable reduction in uric acid compared to 200 mg/kg of AUCF.
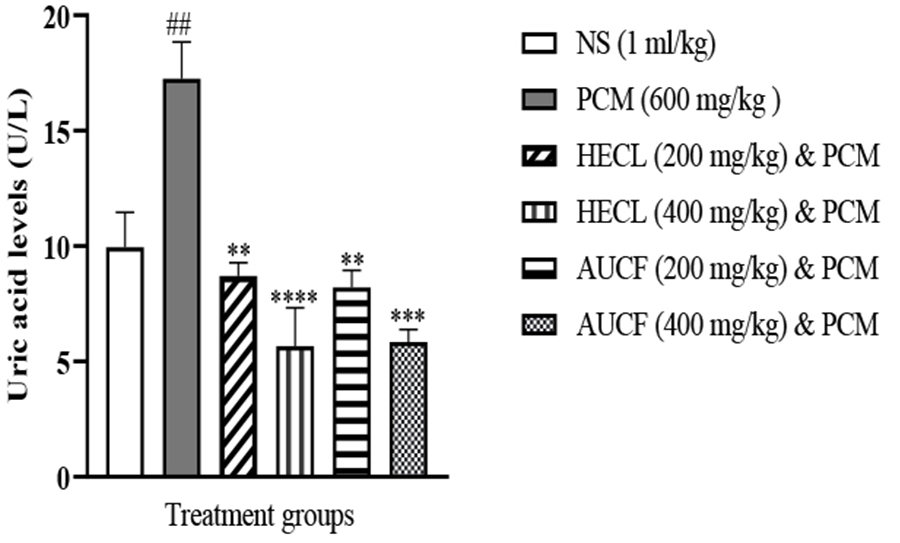 Figure 3. Showing Uric Acid levels across all groups (n = 5). ##p < 0.01 versus N.S, **p < 0.01, ***p < 0.001, ****p < 0.0001 versus PCM. NS: Normal Saline, PCM: Paracetamol, HECL: Hydroethanol C. papaya leaf, AUCF: Aqueous unripe C. papaya fruit
Histopathologic Assessment
Histopathological studies of various treatment groups are displayed in Figure 4. The NS group (Figure 4A) showed normal liver architecture with central vein (CV) and conserved hepatocytes (black arrow). The PCM control group (Figure 4B) showed significant fat deposition, ballooning of hepatocytes (black arrow), necrosis, and periportal infiltration of mononuclear inflammatory cells (red arrow). At the same time, HECL 200 mg/kg and PCM 600 mg/kg, as well as AUCF 200 mg/kg and PCM 600 mg/kg (Figures 4C and 4E, respectively), showed moderate periportal lymphocytic infiltration (red arrowhead). Moreover, HECL 400 mg/kg and PCM 600 mg/kg (Figure 4D) showed mild periportal lymphocytic infiltration. It is noteworthy that AUCF 400 mg/kg and PCM 600 mg/kg (Figure 4F) lacked periportal lymphocytic infiltration (red arrowhead).
Figure 3. Showing Uric Acid levels across all groups (n = 5). ##p < 0.01 versus N.S, **p < 0.01, ***p < 0.001, ****p < 0.0001 versus PCM. NS: Normal Saline, PCM: Paracetamol, HECL: Hydroethanol C. papaya leaf, AUCF: Aqueous unripe C. papaya fruit
Histopathologic Assessment
Histopathological studies of various treatment groups are displayed in Figure 4. The NS group (Figure 4A) showed normal liver architecture with central vein (CV) and conserved hepatocytes (black arrow). The PCM control group (Figure 4B) showed significant fat deposition, ballooning of hepatocytes (black arrow), necrosis, and periportal infiltration of mononuclear inflammatory cells (red arrow). At the same time, HECL 200 mg/kg and PCM 600 mg/kg, as well as AUCF 200 mg/kg and PCM 600 mg/kg (Figures 4C and 4E, respectively), showed moderate periportal lymphocytic infiltration (red arrowhead). Moreover, HECL 400 mg/kg and PCM 600 mg/kg (Figure 4D) showed mild periportal lymphocytic infiltration. It is noteworthy that AUCF 400 mg/kg and PCM 600 mg/kg (Figure 4F) lacked periportal lymphocytic infiltration (red arrowhead).
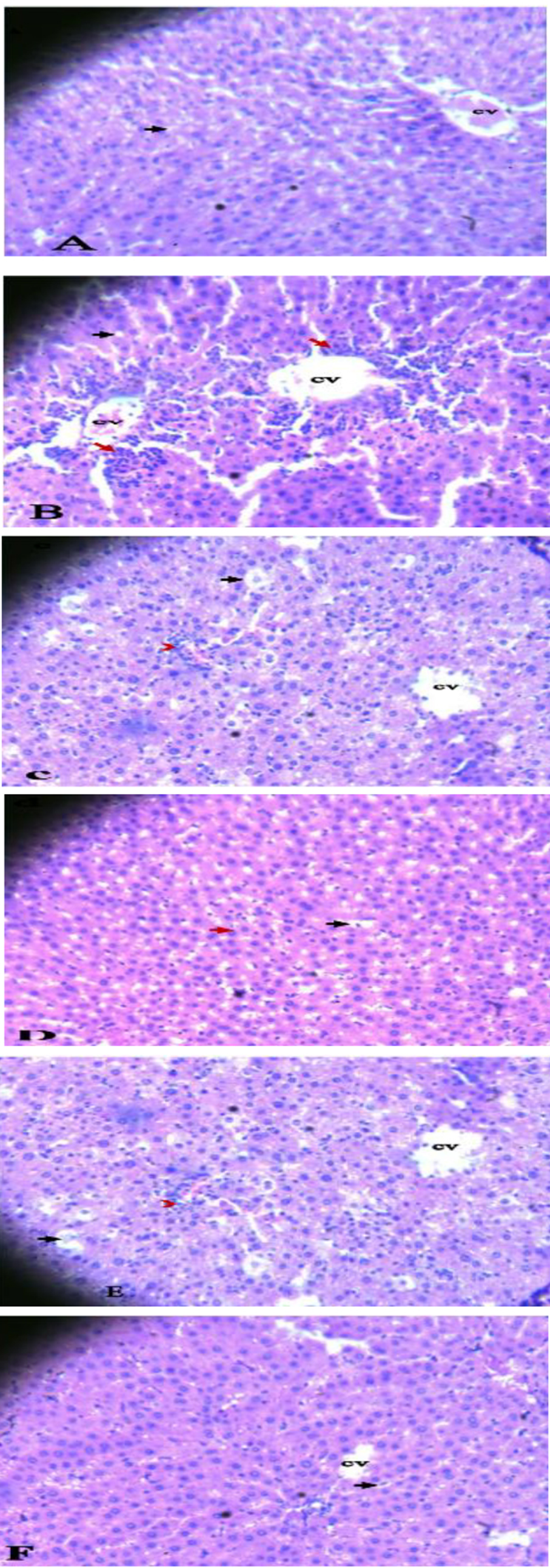 Figures 4. A-F showing photomicrographs of liver tissues in all groups. The NS group (Figure 4A), PCM group (Figure 4B), HECL (200 mg/kg) & PCM and AUCF (200 mg/kg) & PCM (Figures 4C and 4E, respectively). HECL (400 mg/kg) & PCM and AUCF 400 mg/kg & PCM (Figures 4D and 4F, respectively). Central vein (CV); Hepatocytes (Black arrow); inflammatory cells (red arrow). Where NS: Normal Saline (1 ml/kg); PCM: paracetamol (600 mg/kg); HECL: hydroethanol C. papaya leaf; AUCF: aqueous unripe C. papaya fruit
Discussion
Comparisons of the antihepatotoxic activities of the hydroethanol leaf and aqueous fruit extract of C. papaya against PCM-induced hepatic injury were studied in rats. Orally administered PCM, at high doses, is an extensively used model for the screening of hepatoprotective agents [21]. Its mechanism is through the bioactivation of its metabolite, NAPQI, by cytochrome p450 [22]. This alkylating metabolite covalently binds to cellular proteins, which depletes glutathione stores and polyunsaturated fatty acids, initiates oxidative stress and lipid peroxidation. These chemical processes adversely affect the permeability of plasma membranes to Ca+, resulting in hepatocellular damage and necrosis and leakage of liver enzymes, such as ALT, AST, and uric acid, into the serum [17]. Therefore, the estimation of these liver enzyme concentrations in the serum remains a valid index of the extent of the liver injury [23].
The oral acute toxicity test revealed that the leaf and fruit extracts of C. papaya were both safe at a maximum dose of approximately 5 g/kg [18].
Oral administration of 600 mg/kg PCM elicited significant hepatotoxicity with elevations in serum ALT, AST, and uric acid levels in control rats. Increased serum ALT level was particularly indicative of hepatocellular injury [24], of which HECL and AUCF significantly decreased at doses of 200 and 400 mg/kg, respectively, when compared with the control, indicating its hepatocellular regeneration. However, 400 mg/kg of each extract produced a significant decrease in ALT compared to a dose of 200 mg/kg (P<0.001). A significant reduction in serum ALT level by HECL compared to the same dose of AUCF demonstrates that HECL has significantly greater hepatocellular healing effects compared to the same doses of AUCF (Figure 1). This finding contrasts with a previous study [5], suggesting that the hydroethanol leaf extract used in this work may have higher bioactive phytochemicals than the ethanol extract of C. papaya employed in the previous investigation.
Similarly, increased serum AST level following the oral administration of 600 mg/kg PCM is indicative of necrotic hepatocytes, which was most significantly reduced in groups administered 400 mg/kg of HECL than AUCF, indicating that HECL is a more potent anti-necrotic agent than AUCF. Notably, the aqueous seed extract of C. papaya produced a significant antinecrotic activity at a dose of 200 mg/kg, as reported by a worker [25]. This could partly explain the antinecrotic activity observed for AUCF, being a combination of both fruit and seeds. Furthermore, Khor et al. demonstrated that increased level of serum uric acid is significantly associated with non-alcoholic fatty liver disease, of which HECL and AUCF significantly reduced its serum concentration, with a significant reduction of serum uric acid at 400 mg/kg HECL and AUCF (P<0.001). This finding demonstrates that both HECL and AUCF produced hepatoprotective activity capable of preventing non-alcoholic fatty liver disease [26]. These ameliorations or repairs of the liver were further corroborated by the histopathological findings in the hepatic histoarchitecture of treated rats.
Further explanations of the anti-hepatoprotective activities recorded in this study can be attributed to the abundance of potent antioxidative phytochemicals and nutrients recorded in parts of the C. papaya plant. These include alkaloids, flavonoids, phenolics, tannins, saponins, anthraquinones, terpenoids, papain, cystatin, cyanogenic glucosides, glucosinolates, vitamin A, vitamin C, vitamin E, minerals, and pantothenic acids, which can boost the total antioxidant power in the blood and reduce lipid peroxidation levels [27,28] Furthermore, the reducing and free radical scavenging properties of the C. papaya extracts have been linked to the bioactivities for hepatocellular protection [29,30].
Histological examination of liver tissue in rats treated with PCM alone expressed marked hepatocellular necrosis, fatty cell accumulation, inflammatory cell infiltration and other manifestations that are consistent with the alteration in serum liver enzymes, but were markedly reduced upon co-treatment with leaf and fruit extracts of C. papaya with the most significant restoration observed in the groups treated with 400 mg/kg of both the leaf and fruit extracts, in compliance with Awodele et al [5].
Conclusions
The results obtained from the biochemical analyses and confirmed by the histopathological examination revealed that the hydroethanol leaf and aqueous unripe fruit extracts of C. papaya showed equivalent antihepatotoxic activity against PCM-induced hepatic injury in rats, of which bioactive phytochemicals and specific underlying mechanisms require further elucidation.
Limitations
The study lacked data on the isolation and identification of the specific active constituents responsible for the antihepatoprotective effect.
Ethical Considerations
This study adhered to the National Institute of Health (NIH) guide for the Care and Use of Laboratory Animals. It was approved by the Animal Ethical Research Committee (AERC) of Lagos State University (Reference No. AERC/2022/090).
Authors' Contributions
Research conceptualization was made by Amole OA and Yemitan OK. Experimental execution and sample collection were conducted by Arinze KV and Ojewale AO. Organ isolation, laboratory analysis was conducted by Dada AO, Ojewale AO, while histopathological assessment was done by Emiogun EF. Manuscript writing and analysis were done by Arinze KV, while the review was conducted by Yemitan OK, Amole OA, Dada AO, Ojewale AO, Emiogun EF
Acknowledgement
We would like to extend our sincere gratitude to the Departments of Pharmacology, Therapeutics and Toxicology, and Chemical Pathology, Lagos State University College of Medicine, Ikeja, Nigeria, for the provision of kits and other analytical reagents used in this work.
Conflict of Interests
The authors declare that there is no conflict of interest.
Funding
The study did not receive any funding.
Figures 4. A-F showing photomicrographs of liver tissues in all groups. The NS group (Figure 4A), PCM group (Figure 4B), HECL (200 mg/kg) & PCM and AUCF (200 mg/kg) & PCM (Figures 4C and 4E, respectively). HECL (400 mg/kg) & PCM and AUCF 400 mg/kg & PCM (Figures 4D and 4F, respectively). Central vein (CV); Hepatocytes (Black arrow); inflammatory cells (red arrow). Where NS: Normal Saline (1 ml/kg); PCM: paracetamol (600 mg/kg); HECL: hydroethanol C. papaya leaf; AUCF: aqueous unripe C. papaya fruit
Discussion
Comparisons of the antihepatotoxic activities of the hydroethanol leaf and aqueous fruit extract of C. papaya against PCM-induced hepatic injury were studied in rats. Orally administered PCM, at high doses, is an extensively used model for the screening of hepatoprotective agents [21]. Its mechanism is through the bioactivation of its metabolite, NAPQI, by cytochrome p450 [22]. This alkylating metabolite covalently binds to cellular proteins, which depletes glutathione stores and polyunsaturated fatty acids, initiates oxidative stress and lipid peroxidation. These chemical processes adversely affect the permeability of plasma membranes to Ca+, resulting in hepatocellular damage and necrosis and leakage of liver enzymes, such as ALT, AST, and uric acid, into the serum [17]. Therefore, the estimation of these liver enzyme concentrations in the serum remains a valid index of the extent of the liver injury [23].
The oral acute toxicity test revealed that the leaf and fruit extracts of C. papaya were both safe at a maximum dose of approximately 5 g/kg [18].
Oral administration of 600 mg/kg PCM elicited significant hepatotoxicity with elevations in serum ALT, AST, and uric acid levels in control rats. Increased serum ALT level was particularly indicative of hepatocellular injury [24], of which HECL and AUCF significantly decreased at doses of 200 and 400 mg/kg, respectively, when compared with the control, indicating its hepatocellular regeneration. However, 400 mg/kg of each extract produced a significant decrease in ALT compared to a dose of 200 mg/kg (P<0.001). A significant reduction in serum ALT level by HECL compared to the same dose of AUCF demonstrates that HECL has significantly greater hepatocellular healing effects compared to the same doses of AUCF (Figure 1). This finding contrasts with a previous study [5], suggesting that the hydroethanol leaf extract used in this work may have higher bioactive phytochemicals than the ethanol extract of C. papaya employed in the previous investigation.
Similarly, increased serum AST level following the oral administration of 600 mg/kg PCM is indicative of necrotic hepatocytes, which was most significantly reduced in groups administered 400 mg/kg of HECL than AUCF, indicating that HECL is a more potent anti-necrotic agent than AUCF. Notably, the aqueous seed extract of C. papaya produced a significant antinecrotic activity at a dose of 200 mg/kg, as reported by a worker [25]. This could partly explain the antinecrotic activity observed for AUCF, being a combination of both fruit and seeds. Furthermore, Khor et al. demonstrated that increased level of serum uric acid is significantly associated with non-alcoholic fatty liver disease, of which HECL and AUCF significantly reduced its serum concentration, with a significant reduction of serum uric acid at 400 mg/kg HECL and AUCF (P<0.001). This finding demonstrates that both HECL and AUCF produced hepatoprotective activity capable of preventing non-alcoholic fatty liver disease [26]. These ameliorations or repairs of the liver were further corroborated by the histopathological findings in the hepatic histoarchitecture of treated rats.
Further explanations of the anti-hepatoprotective activities recorded in this study can be attributed to the abundance of potent antioxidative phytochemicals and nutrients recorded in parts of the C. papaya plant. These include alkaloids, flavonoids, phenolics, tannins, saponins, anthraquinones, terpenoids, papain, cystatin, cyanogenic glucosides, glucosinolates, vitamin A, vitamin C, vitamin E, minerals, and pantothenic acids, which can boost the total antioxidant power in the blood and reduce lipid peroxidation levels [27,28] Furthermore, the reducing and free radical scavenging properties of the C. papaya extracts have been linked to the bioactivities for hepatocellular protection [29,30].
Histological examination of liver tissue in rats treated with PCM alone expressed marked hepatocellular necrosis, fatty cell accumulation, inflammatory cell infiltration and other manifestations that are consistent with the alteration in serum liver enzymes, but were markedly reduced upon co-treatment with leaf and fruit extracts of C. papaya with the most significant restoration observed in the groups treated with 400 mg/kg of both the leaf and fruit extracts, in compliance with Awodele et al [5].
Conclusions
The results obtained from the biochemical analyses and confirmed by the histopathological examination revealed that the hydroethanol leaf and aqueous unripe fruit extracts of C. papaya showed equivalent antihepatotoxic activity against PCM-induced hepatic injury in rats, of which bioactive phytochemicals and specific underlying mechanisms require further elucidation.
Limitations
The study lacked data on the isolation and identification of the specific active constituents responsible for the antihepatoprotective effect.
Ethical Considerations
This study adhered to the National Institute of Health (NIH) guide for the Care and Use of Laboratory Animals. It was approved by the Animal Ethical Research Committee (AERC) of Lagos State University (Reference No. AERC/2022/090).
Authors' Contributions
Research conceptualization was made by Amole OA and Yemitan OK. Experimental execution and sample collection were conducted by Arinze KV and Ojewale AO. Organ isolation, laboratory analysis was conducted by Dada AO, Ojewale AO, while histopathological assessment was done by Emiogun EF. Manuscript writing and analysis were done by Arinze KV, while the review was conducted by Yemitan OK, Amole OA, Dada AO, Ojewale AO, Emiogun EF
Acknowledgement
We would like to extend our sincere gratitude to the Departments of Pharmacology, Therapeutics and Toxicology, and Chemical Pathology, Lagos State University College of Medicine, Ikeja, Nigeria, for the provision of kits and other analytical reagents used in this work.
Conflict of Interests
The authors declare that there is no conflict of interest.
Funding
The study did not receive any funding.
References
- Emeje M, Oppong BE, Graz B and Willcox M. Traditional medicine development in Africa: Opinion. J Integr Complement Med. 2023;6:340-343. [DOI: 10.1089/jicm.2022.0825] [PMID: 37036786]
- Burns P, Saengmanee P, Doung-Ngern U. Papaya: The versatile tropical fruit. Tropical Plant Species and Technological Interventions for Improvement. IntechOpen; 2023. [DOI: 10.5772/intechopen.104624]
- Oduola T, Adeniyi AA, Ogunyemi EO, Bello IS, Idowu TO. Antisickling agent in an extract of unripe pawpaw (Carica papaya L.): Is it real? Afr J Biotech. 2006;5(20):1947-1949. [Link]
- Otsuki N, Dang N, Kumagai E, Kondo A, Iwata S, Morimoto C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethnopharmacol. 2010;127:760-767. [DOI: 10.1016/j.jep.2009.11.024] [PMID: 19961915]
- Awodele O, Yemitan OK, Ise PU, Ikumawoyi VO. Modulatory potentials of aqueous leaf and unripe fruit extracts of Carica papaya Linn. (Caricaceae) against carbon tetrachloride and acetaminophen-induced hepatotoxicity in rats. J Intercult Ethnopharmacol. 2016;5(1):27-35. [DOI: 10.5455/jice.20160124113528] [PMID: 27069723]
- Shaban NZ, El-Kot SM, Awad OM, Hafez AM, Fouad GM. The antioxidant and anti-inflammatory effects of Carica papaya Linn. seeds extract on CCl4-induced liver injury in male rats. BMC Complement Med Ther. 2021;21(1):302. [DOI:10.1186/s12906-021-03479-9] [PMID: 34969385]
- Pambhar V, Mathur N, Mehta A, Mathur M, Kumawat DC, Mangalia R, et al. Effect of doxycycline and doxycycline with Carica papaya on thrombocytopenia and leucopenia in acute dengue fever patients. J Fam Med Prim Care. 2022;11(6):3270–3275. [DOI: 10.4103/jfmpc.jfmpc_53_22] [PMID: 36119216]
- Ghosh S, Saha M, Bandyopadhyay PK, Jana M. Extraction, isolation and characterization of bioactive compounds from chloroform extract of Carica papaya seed and it’s in vivo antibacterial potentiality in Channa punctatus against Klebsiella PKBSG14. Microb pathog. 2017;111:508–518. [DOI: 10.1016/j.micpath.2017.08.033] [PMID: 28867632]
- Harmayani E, Anal AK, Wichienchot S, Bhat R, Gardjito M, Santoso U, et al. Healthy food traditions of Asia: Exploratory case studies from Indonesia, Thailand, Malaysiaand Nepal. J Ethn Food. 2019;6:1. [DOI:10.1186/s42779-019-0002-x]
- Sai K, Thapa R, Devkota HP, Joshi KR. Phytochemical screening, free radical scavenging and α-amylase inhibitory activities of selected medicinal plants from western Nepal. Medicines (Basel). 2019;6(2):70. [DOI: 10.3390/medicines6020070] [PMID: 31242563]
- Alchin J, Dhar A, Siddiqui K, Christo PJ. Why paracetamol (acetaminophen) is a suitable first choice for treating mild to moderate acute pain in adults with liver, kidney or cardiovascular disease, gastrointestinal disorders, asthma, or who are older. Curr Med Res Opin. 2022;38(5):811–825. [DOI: 10.1080/03007995.2022.2049551] [PMID: 35253560]
- Amadi CN, Orisakwe OE. Herb-induced liver injuries in developing nations: An Update. Toxics. 2018;6(2):24. [DOI: 10.3390/toxics6020024] [PMID: 29673137]
- Rani J, Dhull SB, Rose PK, Kidwai MK. Drug-induced liver injury and antihepatotoxic effect of herbal compounds: a metabolic mechanism perspective. Phytomedicine. 2024;122,155142. [DOI: 10.1016/j.phymed.2023.155142] [PMID: 37913641]
- Cai X, Cai H, Wang J, Yang Q, Guan J, Deng J, et al. Molecular pathogenesis of acetaminophen-induced liver injury and its treatment options. J Zhejiang Univ Sci B. 2022;23(4):265–285. [DOI: 10.1631/jzus.B2100977] [PMID: 35403383]
- Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med. 2016;14:10. [DOI: 10.1186/s12916-016-0553-2] [PMID: 26843061]
- Ladele AA, Bisi-Amosun OO. Level of utilization of traditional and orthodox medicines by rural dwellers in ileOgbo community of Osun State, Nigeria. J Agricul Exten. 2014,18(1):155-168. [DOI:10.4314/jae.v18i1.14]
- Doumbia S, Denou A, Haidara M, Dembele D, Fofie N, Sanogo R. A review on medicinal plants used in the management of liver diseases in west Africa. J Pharm Bioresources. 2023;20(2):56-73. [DOI.10.4314/jpb.v20i2.1]
- OECD. Guidance document on acute oral toxicity testing. OECD Series on Testing and Assessment;No. 24. OECD Publishing, Paris; 2002 [DOI:10.1787/9789264078413-en]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. [DOI: 10.1093/ajcp/28.1.56] [PMID: 13458125]
- Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol.1960;13(2):156–159. [DOI: 10.1136/jcp.13.2.156] [PMID: 13821779]
- Nimbalkar VV, Pansare PM, Nishane BB. Screening methods for hepatoprotective agents in experimental animal's. Res J Pharm Technol. 2015;8:1725-1732. [DOI:10.5958/0974-360X.2015.00310.8]
- Nayak SS, Jain R, Sahoo AK. Hepatoprotective activity of glycosmis pentaphylla against paracetamol-induced hepatotoxicity in Swiss albino mice. Pharm Biol. 2011;49(2):111-117. [DOI: 10.3109/13880209.2010.501084] [PMID: 20942640]
- Garba SH, Sambo N, Bala U. The effect of the aqueous extract of Kohautia grandiflora on paracetamol-induced liver damage in albino rats. Niger J Physiol Sci. 2009;24(1):17-23. [DOI: 10.4314/njps.v24i1.46376] [PMID:19826460]
- Li C, Yi LT, Geng D, Han YY, Weng LJ. Hepatoprotective effect of ethanol extract from Berchemia lineate against CCl4-induced acute hepatotoxicity in mice. Pharm Biol. 2015;53(5):767-772. [DOI: 10.3109/13880209.2014.941506] [PMID:25431325]
- Naggayi M, Mukiibi N, Iliya E. The protective effects of aqueous extract of Carica papaya seeds in paracetamol induced nephrotoxicity in male wistar rats. Afr Health Sci. 2015;15(2):598–605. [DOI: 10.4314/ahs.v15i2.37] [PMID: 26124809]
- Khor BK, Chear NJ, Azizi J, Khaw KY. Chemical composition, antioxidant and cytoprotective potentials of Carica papaya leaf extracts: A comparison of supercritical fluid and conventional extraction methods. Molecules. 2021;26(5):1489. [DOI: 10.3390/molecules26051489]
- Eke ON, Augustine AU, Ibrahim HF. Qualitative analysis of phytochemicals and antibacterial screening of extracts of Carica papaya fruits and seeds. Int J Modern Chem. 2014;6 (1):48-56. [Link]
- Husin F, Ya’akob H, Rashid SNA, Shahar S, Soib HH. Cytotoxicity study and antioxidant activity of crude extracts and SPE fractions from Carica papaya leaves. Biocataly Agric Biotechnol. 2019;19:1–6. [DOI:10.1016/j.bcab.2019.101130]
- Jarisarapurin W, Sanrattana W, Chularojmontri L, Kunchana K, Wattanapitayaku SK. Antioxidant properties of unripe Carica papaya fruit extract and its protective effects against endothelial oxidative stress. Evid Based Complement Alternat Med. 2019;2019:4912631. [DOI: 10.1155/2019/4912631] [PMID: 31320913]
- Asjad HMM, Akhtar MS, Khan M, Sial NT, Sohail I, Arshad L. Ethanolic extract of Citrus sinensis peel markedly mitigate paracetamol-induced hepatotoxicity in rats. J Health Rehabil Res. 2023;3(2):1058-1062. [DOI:10.61919/jhrr.v3i2.270]
Type of Study:
Research |
Subject:
Special










































 , Olufemi Amole1
, Olufemi Amole1 
 , Adeyemi Dada2
, Adeyemi Dada2 

 , Abdulfatai Ojewale3
, Abdulfatai Ojewale3 

 , Edobor Emiogun4
, Edobor Emiogun4 

 , Omoniyi Yemitan *5
, Omoniyi Yemitan *5 






