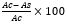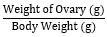Introduction
The PCOS is a heritable illness that affects women’s entire lives, leading to symptoms, such as amenorrhea, oligo-amenorrhea, hirsutism, enlarged ovaries with multiple cysts, and obesity [1]. These symptoms may be triggered by abnormal androgen metabolism and production during the reproductive years. Lack of exercise, high-calorie diet, obesity, and insulin resistance have been considered as major contributing factors for PCOS [2]. PCOS affects 6-12% of women in the reproductive phase globally and is one of the most commonly investigated syndromes, which has been assessed in all regions of the world [3]. The prevalence of PCOS varies worldwide, ranging from 2.2% to 26%. In Asian countries, the reported rates range from 2% to 7.5% in China and 6.3% in Sri Lanka. In India, the prevalence is notably higher, with rates of 9.13% in Maharashtra and 22.5% in southern India [4]. As such, no definite treatment is available for the cure of PCOS, and allopathic drugs available only provide symptomatic treatment and are also associated with various side effects [5] Recent studies found that herbal therapies are quite effective in the treatment of PCOS, and various natural products have been regularly used as dietary supplements by patients with PCOS [6].
Cornus capitata Wall. (Cornaceae) fruits commonly known as bhamora or Himalayan strawberry (Figure 1) have been used traditionally for the cure of obesity and diabetes [7]. Fruits contain biological effects like anti-inflammatory, antibacterial, anticancer, and antioxidant [8]. Leaves have been demonstrated to have antiviral, antidiabetic, antihyperlipidemic, and semen-coagulating properties [9]. MECCF elucidated a prominent inhibitory activity against the α-glucosidase enzyme, having traditional use in managing diabetes, which ultimately reduces high blood pressure, high cholesterol levels, and obesity [10,11]. Steam, flowers, and leaves of this plant contain terpenoids (Arjunolic acid), flavonoids, coumarins, polyphenols, and glycosides, which are known to be major phytoconstituents in the management of PCOS symptoms [10,12].
Zebrafish (Danio rerio) is a 2–5 cm in size (Figure 2) striped tropical fish that shares 70% of its genome with humans, and more than 80% of disease proteins are found, especially those involved in drugs that interact with target proteins [13]. Nowadays, freshwater teleost like Zebrafish is a widespread animal model due to their high fertility (at least 200 eggs per clutch), transparent embryos, fewer space requirements, and low maintenance for zebrafish husbandries. Zebrafish can absorb any water-soluble drug through their skin and gills, which makes drug delivery much easier [14]. The transparent embryos grow rapidly and are easy to visualize and genetically manipulate [15]. The ovarian regulatory mechanisms of zebrafish and humans are strikingly similar [16]. The Zebrafish model could serve as an alternative to using mammals in research, and helps address concerns related to animal welfare [17]. Zebrafish is considered the potential model in infertility research because of the remarkable similarities with mammals in reproductive functions [18]. Short life span, several offspring, and clear growing phases favor toxicity studies of substances, such as pesticides, nanoparticles, and different organic toxins in the atmosphere [6].
Therefore, the present study aimed to study the toxicity profiling and therapeutic effects of the methanolic extract of Cornus capitata Well. fruits (MECCF) using a fish embryo toxicity assay and a testosterone-induced PCOS in a zebrafish model.

Figure 1. An image of fruit and leaves of Cornus capitata Wall.

Figure 2. A photo of adult zebrafish.
Materials and Methods
Materials
High-purity chemicals were procured for the study. Methanol, 2, 2-diphenyl-1-picrylhydrazyl, ferric chloride, and trolox were obtained from Sigma-Aldrich. Testosterone and dimethyl sulfoxide were obtained from Zydus Healthcare Ltd.
Collection and authentication of plant material
The Cornus capitata wall. fruits were collected from Rudraprayag, Uttarakhand region, during September 2022. The plant was authenticated by the Botanical Survey of India, Dehradun, Uttarakhand.
Preparation of the methanolic extract of Cornus capitata Wall. fruit (MECCF)
Cornus capitata Wall. fruits were washed, shade-dried, and crushed with the help of an electric blender. The Soxhlet apparatus was used to extract the fruit (50 g) using 250 mL of methanol (99.5% v/v) as the solvent, operating continuously for 24 hours with monitoring. The extract was concentrated at 50°C–60°C under reduced pressure using a rotary evaporator and was stored at 4°C [19].
The extract was subjected to various qualitative phytochemical screening of MECCF for the determination of the nature of phytoconstituents present in it [20].
In vitro Antioxidant Assay of MECCF
DPPH Antioxidant Assay: To prepare the reaction mixture, 1 mL of 0.1 mM DPPH solution was added to a 10 mL volumetric flask containing the extract dissolved in methanol (99.5% v/v) at varying concentrations (50 µg/mL to 500 µg/mL). The mixture was thoroughly mixed and incubated at room temperature for 30 minutes. Methanol was used as the blank, and the absorbance was recorded at 517 nm. Ascorbic acid served as the standard [21]. The test was performed in triplicate. Radical scavenging activity was stated in terms of % inhibition and was calculated with the formula:
% RSA= 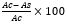
Ferric Reducing Antioxidant Power (FRAP) Assay: An amount of 3 ml of FRAP reagent (10 parts of 300 mM sodium acetate buffer at pH 3.6, 1 part of 10 mM TPTZ solution, and 1 part of 20 mM FeCl3.6H2O solution) was mixed with fruit extract, and the reaction mixture was incubated at 37°C for 30 minutes. The absorbance of the reaction mixture was measured at 593 nm. µmol ascorbic acid equivalents per gram of plant material were used to express the antioxidant capability [22].
Animals: The study protocol was approved by the Institutional Animal Ethical Committee of Siddhartha Institute of Pharmacy (SIP/IAEC/PCOL/16/2022) in accordance with the requirements of the CPCSEA, Government of India, for conducting animal studies. Zebrafish (3 and 8 months of age, AB wild-type of 3.7–4 cm long, 470-550 mg weight) were maintained in the 5 L tanks (40 fish per tank, males and females were intermixed) in distilled water (pH- 7.0±0.2, temp- 28±0.5 °C), and well-lit with fluorescent ceiling light in 14:10 light-dark cycles (light on, 7 AM; light off, 9 PM). Every day, about 10% of the entire volume was replenished. The sexual dimorphisms of the fish, such as body shape and skin color, were used to distinguish between males and females [23].
Fish Embryo Acute Toxicity Study: Fish embryo acute toxicity studies were conducted in accordance with OECD Guideline 236 [24]. For a week, the male and female fish were raised apart. For spawning, the fish are kept in a breeding tank in a 5:3 ratio (female: male). The fish were left undisturbed overnight in the dark. Fish began laying eggs after being exposed to light. To prevent contamination, the eggs were promptly retrieved and cleaned with 0.0001% methylene blue. After washing the embryo media twice, the egg’s viability was examined under a microscope [25]. A 24-well plate containing freshly prepared plant extract at several doses (5, 2.5, 1.25, 0.625, 0.3125, and 0.156 g/L) or E3 embryo media as a control condition was used to transfer viable eggs, <3-hour post fertilization (hpf). The solution was changed daily until 96 hpf, and a total of 20 embryos were used for each condition (five embryos in 1 ml/well). The embryos were examined every day to assess somite formation and coagulation as markers of aberrant development and/or mortality under a microscope [26].
Testosterone-induced PCOS Treatment: An amount of 100ng Testosterone was dissolved in distilled water containing 0.1% dimethyl sulfoxide and stored at −20 °C. Eight female zebrafish were placed in each tank and allowed to swim for 3 days under standard rearing conditions (14:10 light-dark cycle, 28°C, regular feeding), with the drug solutions being replaced daily. Fish should not exhibit any symptoms of stress or discomfort, such as jerky movements or motionless behavior, during treatment at the bottom of the container. The control was given 0.1% DMSO in distilled water [23].
Zebrafish were divided into three groups (n=6) and treated with different concentrations of MECCF for 7 days.
Group I (Positive control): Fishes with PCOS+0.1% DMSO in distilled water.
Group II (Treatment group I): Fishes with PCOS+31.25 mg/L MECCF1.
Group III (Treatment group II): Fishes with PCOS+62.25 mg/L MECCF2.
Evaluation Parameters
- Measurement of the Testosterone Levels: The Zebrafish was euthanized with 1% tricaine mesylate mixed in water at 4°C. Ovary and brain tissues were isolated, weighed, and homogenized with methanol (75% in water; 1 µg tissue in 1 µl solution). The sample was centrifuged at 2000 g at 4°C, and the supernatant was collected. The process was repeated three times. The supernatant was combined, lyophilized, and stored at − 80°C for the measurement of testosterone levels [23].
- Measurement of the Gonadosomatic Index: This index is used to evaluate the growth and function of the ovaries. Fishes were anaesthetized, wiped, sacrificed, and the ovaries were dissected and weighed [23].
GSI= 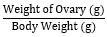
- Histology of Ovary: The dissected ovaries were fixed in 4% paraformaldehyde at 4°C overnight. The tissue specimens were sequentially dehydrated in 80%, 90%, 95%, and 100% ethanol, followed by xylene, and then embedded in paraffin before being stored at -20 °C. Moreover, 6-µm-thick slices were collected, placed on glass slides, allowed to dry naturally, stained with hematoxylin and eosin, and covered with glass coverslips. Eight sections were taken at 6-mm intervals from each ovary. The sections were examined under a microscope, and the size and location of endocrine components in the surrounding tissues were used as markers to assess the development, transition, and maturation of the follicles [23].
Statistical analysis
The statistical analysis was carried out using GraphPad Prism 9.0 software. All values were presented as mean±SEM. Comparison between the two groups was performed using Student's t-test. Multiple comparisons between different groups were performed using analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. A difference level at P<0.05 was considered statistically significant.
Results
The effect of MECCF was evaluated in testosterone-induced PCOS in zebrafish. Toxicity profiling of the extract was also carried out in zebrafish. Phytochemical studies revealed that the % yield of MECCF after Soxhlet extraction was found to be 18.84%.
Phytochemical analysis
The phytochemical investigation of the MECCF revealed the presence of terpenoids, polyphenols (including flavonoids), proteins, carbohydrates, tannins, and Saponins.
Evaluation of antioxidant activity of MECCF by DPPH method
Results in Figure 3 indicate the free radical scavenging effect of MECCF and ascorbic acid by the DPPH method. The result demonstrates that polyphenols and flavonoids present in MECCF exhibited a dose-dependent increase in the scavenging of free radicals, as plotted in Figure 3. The correlation coefficient (R2) was found to be 0.9371 for MECCF and 0.9749 for Ascorbic acid.
Ferric reducing antioxidant power (FRAP) assay
Antioxidant assay of MECCF was measured by using FRAP reagent. Increase in absorbance is measured at 593 nm. Reducing power expressed the antioxidant capacity as µmol of Ascorbic acid equivalents per gram of MECCF, plotted in Figure 4. The correlation coefficient (R2) of MECCF was found to be 0.9572, and that of Ascorbic acid was found to be 0.9712.
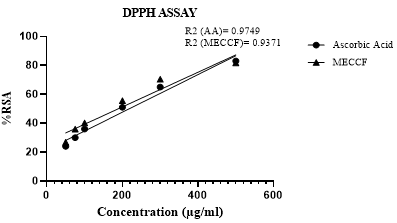
Figure 3. Scavenging effect of MECCF by the DPPH assay.
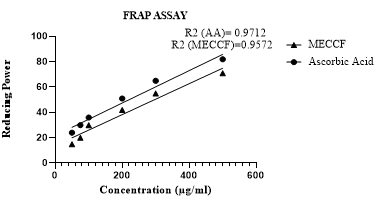
Figure 4. Reducing the power of MECCF by the FRAP.
Fish embryo acute toxicity (FET) test
Morphological analyses of embryos demonstrated that at 5 g/L, 2.5 g/L, and 1.25 g/L, all embryos exhibited coagulation. At a concentration of 0.625 g/L, some embryos exhibited malformations, including egg coagulation. However, at lower concentrations (0.3125 g/L and 0.156 g/L), no developmental abnormalities were observed after 24 hpf, as detailed in Figure 5. The average survivability rate of embryos at various concentrations after 96 hpf of MECCF is presented in Figure 6.
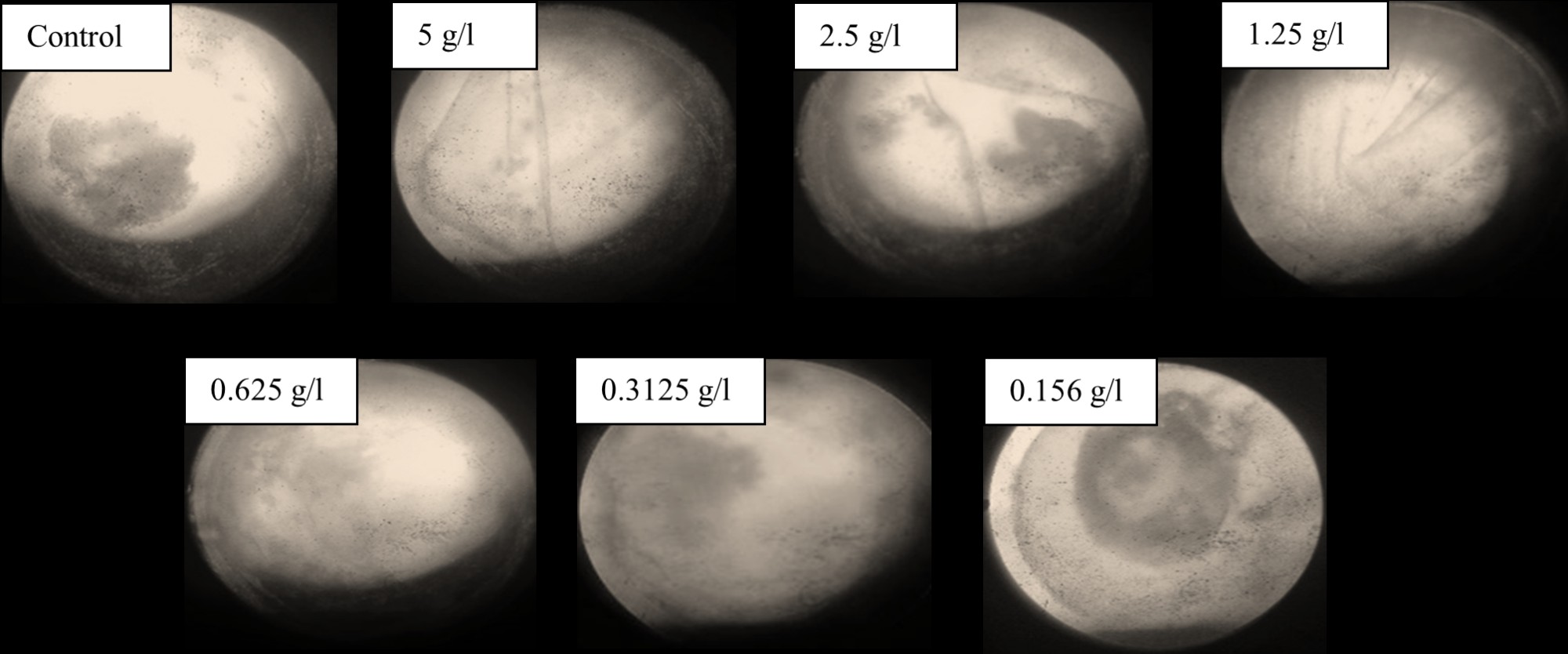
Figure 5. Survival Rate of Fish Embryo in different concentrations of MECCF and Control after 24 hpf. In the control group, all the embryos were viable, and they survived. At a concentration of 5 g/L, the embryos showed coagulation and could not survive. At a concentration of 2.5 g/L, the embryos showed coagulation and could not survive. 1.25 g/l embryos demonstrated coagulation and could not survive. 0.625 g/l embryos indicated malformations and could not survive. 0.3125 g/l embryos were viable and survived. 0.156 g/l embryos were viable and survived.
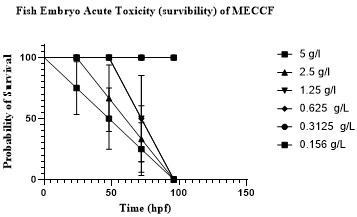
Figure 6. Fish embryo acute toxicity (survivability) of MECCF.
Effect of MECCF on testosterone-induced PCOS in zebrafish
Figures 5 and 6 reveal the impact of MECCF on the level of testosterone, gonadosomatic index, and ovarian histology in zebrafish with PCOS. The testosterone level in PCOS Zebrafish treated with MECCF1 and MECCF2 was analyzed. MECCF2 exhibits a more pronounced reduction in testosterone levels compared to MECCF1 and the control, as shown in Figure 7a. For the calculation of GSI, zebrafish ovaries were extracted and weighed before histopathological analysis. The abdomen was cut lengthwise, the ovarian tissues were removed, weighed, and fixed in 4% formalin.% GSI has been indicated in Figure 7b. Results in Figure 8 show that zebrafish in the control group revealed several ovarian follicles in different stages of development, with developing follicles, transitioning follicles, and mature follicles, which shows decreased corpus luteum and a rise in subcapsular cyst development, capsular thickness, and theca cell hyperplasia. The ovaries of MECCF1-treated zebrafish show few ovarian follicles and corpus luteum with fewer transitioning follicles and mature follicles. The ovarian section of MECCF2-treated zebrafish shows a significant reduction in the transitioning phase and more prominent mature follicles. (P<0.0001).
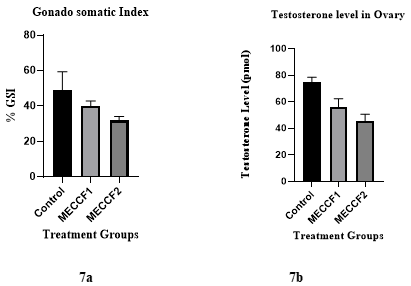
Figure 7. Levels of hormones investigated as a result of AlP poisoning and treatment with different doses of antidotes.
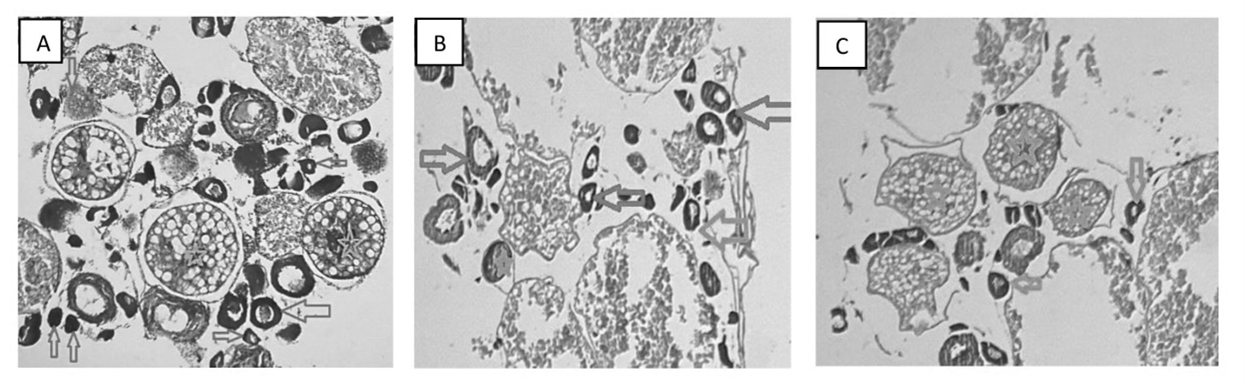
Figure 8. In control fish (A), developing follicles (Stages I and II, red and green arrows), transitioning follicles (Stage III, arrows), and mature follicles (Stages IV and V, stars), show a normal ovary. In treatment Group 1 (B), given MECCF1, transitioning follicles (Stage III, arrows), and mature follicles (Stages IV and V, stars) indicate a MECCF1-treated ovary. In the treatment Group 2 (C), given MECCF2, transitioning follicles (Stage III, arrows), and mature follicles (Stages IV and V, stars), demonstrate a MECCF2-treated normal ovary.
Discussion
Cornus capitata Wall. Extract is traditionally used to manage symptoms of PCOS based on its antioxidant, antidiabetic, antihyperlipidemic, and anti-inflammatory properties. Therefore, this medicinal plant appears to be a good alternative to manage most of the complications associated with PCOS, including obesity and diabetes [10]. In this study, Cornus capitata wall. fruits have been collected from Rudrprayag, Uttarakhand region, the wide ecological growing temperature conditions, and altitudes between 1000 and 7500 m above sea level, which provide quality-rich chemical constituents in medicinal plants. Cornus capitata wall. fruits have been extracted using methanol as a solvent [19], giving 18.84 % yield. Phytochemical analysis of MECCF confirms the occurrence of phenols, flavonoids, Protein, tannin, and saponins. DPPH assay and FRAP assay were used to estimate the in vitro free radical Scavenging potential of MECCF. The main compounds showing antioxidant activity are polyphenols, as they possess an aromatic ring that allows for the stabilization and relocation of the unpaired electrons in their structure, thereby facilitating the donation of hydrogen atoms and electrons from their hydroxyl groups. The correlation coefficients (R2) for both assays were found to be 0.9661 and 0.9567.
The Zebrafish model was selected for toxicity and fertility studies, as a young female zebrafish can lay several hundred eggs every other week. The eggs are fertilized in water, and the transparent embryos develop externally, making them an excellent model for toxicity studies. In the Fish Embryo Acute Toxicity (FET) test, embryo survival analyses revealed that embryo survivability decreased with increasing extract concentrations and exposure time. At higher concentrations, embryos showed some malformations (egg coagulation, somite malformation) after 96 hpf. At lower concentrations, no developmental modifications were detected; however, increased dose and exposure time led to the mortality of zebrafish embryos. After the accomplishment of the safe dose, testosterone-induced PCOS in the Zebrafish model was employed to investigate the MECCF in managing PCOS. According to the results, MECCF2 demonstrated a greater reduction in testosterone levels in the ovaries of zebrafish compared to the control group. The GSI displays the effectiveness of MECCF2 with 31.758. The increase of GSI in PCOS fish confirms that high levels of testosterone in females, in comparison with controls, cause molecular and cellular abnormalities in the ovary. The histology of the ovaries also indicates that MECCF2 is more effective in improving ovarian cysts, as an increase in mature follicles leads to ovulation, and mature ovarian follicles were present more than transitional follicles in the Zebrafish ovary.
Conclusions
Cornus capitata Wall. fruits are extensively used in traditional medicine to reduce blood glucose and cholesterol levels. The fruits are rich in various plant bioactives, such as polyphenols, terpenoids, and flavonoids. In the current study, the MECCF demonstrated a significant decrease in testosterone levels and the GSI index in the zebrafish model of PCOS. The multiple offspring and high similarities in reproductive functions between Zebrafish and mammals have endorsed them as a promising model for toxicity studies and infertility research. These findings suggest that MECCF has the potential to manage the symptoms of PCOS and can be further evaluated in a rodent model of PCOS to confirm its therapeutic effect in managing PCOS.
Data Access and Responsibility
The authors confirm that all the data supporting the findings of this study are available within the article. All authors had full access to the data and take responsibility for the integrity and accuracy of the data analysis.
Ethical Considerations
The study was conducted in accordance with ethical guidelines and was approved by the Institutional Animal Ethics Committee of Siddhartha Institute of Pharmacy, Dehradun. Approval No. SIP/IAEC/PCOL/16/2022.
Authors' Contributions
Indu Tewari : Performed the procedure and wrote the entire manuscript.
Veerma Ram: drafted and revised the manuscript.
Sanjay Singh: supervised the entire procedure critically, draft manuscript
Mamta F. Singh: Conceptualized and revised the manuscript critically.
Acknowledgement
The authors are grateful to the management of SBS University, Balawala, Dehradun, India, and Siddhartha Institute of Technology, Dehradun, India, for their technical backing in performing the procedure and writing this research article.
Conflict of Interests
The authors declared that there is no conflict of interest.
Funding
The authors received no external funding for this study.
References
- Sirmans S, Pate K. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. [DOI: 10.2147/CLEP.S37559] [PMID: 24379699]
- Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. 2011;24(4):223–227. [DOI: 10.1016/j.jpag.2011.03.002] [PMID: 21600812]
- Farhadi-Azar M, Behboudi-Gandevani S, Rahmati M, Mahboobifard F, Khalili Pouya E, Ramezani Tehrani F, et al. The prevalence of polycystic ovary syndrome, its phenotypes and cardio-metabolic features in a community sample of Iranian population: Tehran lipid and glucose study. Front Endocrinol (Lausanne). 2022;13. [DOI: 10.3389/fendo.2022.825528] [PMID: 35299965]
- Joshi B, Mukherjee S, Patil A, Purandare A, Chauhan S, Vaidya R. A cross-sectional study of polycystic ovarian syndrome among adolescent and young girls in Mumbai, India. Indian J Endocrinol Metab. 2014;18(3):317-324. [DOI: 10.4103/2230-8210.131162] [PMID: 24944925]
- Cochran L, Nadolny R, Garcia K, Kluglein KA, Yagoda A, Gandhi P, et al. Available treatments and adjunctive therapies for polycystic ovarian syndrome (pcos) patients of reproductive age: a scoping review. Cureus. 2024;16(9):e70501. [DOI: 10.7759/cureus.70501] [PMID: 39479136]
- Zainol Abidin IZ, Fazry S, Jamar NH, Ediwar Dyari HR, Zainal Ariffin Z, Johari AN, et al. The effects of Piper sarmentosum aqueous extracts on zebrafish (Danio rerio) embryos and caudal fin tissue regeneration. Sci Rep. 2020;10:14165. [DOI: 10.1038/s41598-020-70962-7] [PMID: 32843675]
- Khanduri VP, Sukumaran A, Sharma CM. Reproductive biology of Cornus capitata Wall. ex Roxb.: a native species in East Asia. J For Res (Harbin). 2019;30(6):2039–2050. [DOI:10.1007/s11676-018-0779-2]
- Dimri DB, Nigam M, Singh N, Semwal S, Iriti M, Mishra AP. Orchestrating the phytochemical content and bioactivities in the leaf, bark, and fruit extracts of cornus capitata wall. Phyton-Int J Exp Bot. 2025;94(3):1007–27. [DOI:10.32604/phyton.2025.061270]
- Kumari K, Sharma S, Kaushik R. Wild himalayan fig: a nutraceutical under exploited fruit of western himalayan region – a review. Int J Adv Res (Indore). 2017;5(9):833–839. [DOI:10.21474/IJAR01/5395]
- Bhatia A, Singh B, Arora R, Arora S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complement Altern Med. 2019;19(1):74. [DOI: 10.1186/s12906-019-2482-z] [PMID: 30909900]
- Badoni S, Rawat D, Mahato AK, Jangwan NS, Ashraf GM, Alexiou A, et al. Therapeutic potential of cornus genus: navigating phytochemistry, pharmacology, clinical studies, and advanced delivery approaches. Chem Biodivers. 2024;21(8):e202301888. [DOI: 10.1002/cbdv.202301888] [PMID: 38403786]
- Niaz K, Khan F. Analysis of polyphenolics. In: Recent Advances in Natural Products Analysis. Elsevier; 2020:39–197. [DOI:10.1016/B978-0-12-816455-6.00003-2]
- Patton EE, Zon LI, Langenau DM. Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat Rev Drug Discov. 2021;20(8):611–628. [DOI: 10.1038/s41573-021-00210-8] [PMID: 34117457]
- Adhish M, Manjubala I. Effectiveness of zebrafish models in understanding human diseases—A review of models. Vol. 9, Heliyon. Elsevier Ltd; 2023 13;9(3):e14557.
- Sakai C, Ijaz S, Hoffman EJ. Zebrafish models of neurodevelopmental disorders: past, present, and future. Front Mol Neurosci. 2018;11:294. [DOI: 10.3389/fnmol.2018.00294] [PMID: 30210288]
- Li J, Ge W. Zebrafish as a model for studying ovarian development: Recent advances from targeted gene knockout studies. Mol Cell Endocrinol. 2020;507:110778. [DOI: 10.1016/j.mce.2020.110778] [PMID: 32142861]
- Bailone RL, Fukushima HCS, Ventura Fernandes BH, De Aguiar LK, Corrêa T, Janke H, et al. Zebrafish as an alternative animal model in human and animal vaccination research. Lab Anim Res. 2020;36:13. [DOI: 10.1186/s42826-020-00042-4] [PMID: 32382525]
- Hoo JY, Kumari Y, Shaikh MF, Hue SM, Goh BH. Zebrafish: A versatile animal model for fertility research. Biomed Res Int. 2016;2016:9732780. [DOI: 10.1155/2016/9732780] [PMID: 27556045]
- Lamichhane J, Chaudhary R, Subedi S, Bajracharya R, Shrestha TM. Antimicrobial and cytotoxic property of plant extracts from evergreen Himalayan tree, Cornus capitata. J Biomol Reconstruction. 2009; 6( 2):175-182. [Link]
- Sahira Banu K, Cathrine L. General techniques involved in phytochemical analysis. IJARCS. 2015;2(4):25–32. [Link]
- Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of ficus religiosa. Molecules. 2022;27(4):1326. [DOI: 10.3390/molecules27041326] [PMID: 35209118]
- Wong S, Leong L, Williamkoh J. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99(4):775–783. [DOI:10.1016/j.foodchem.2005.07.058]
- Liu C, Yue S, Solarz J, Lee J, Li L. Improving the sexual activity and reproduction of female zebrafish with high testosterone levels. Sci Rep. 2021;11(1):3822. [DOI: 10.1038/s41598-021-83085-4] [PMID: 33589678]
- OECD. OECD Guidelines for the Testing of Chemicals 236, Fish embryo acute toxicity (FET) Test. 24. OECD;2025. [DOI:10.1787/9789264203709-en]
- R. EB, Jesubatham PD, Berlin BG, Viswanathan S, Srividya S. Non-toxic and non teratogenic extract of Thuja orientalis L. inhibited angiogenesis in zebra fish and suppressed the growth of human lung cancer cell line. Biomed Pharmacother. 2018;106:699–706. [DOI: 10.1016/j.biopha.2018.07.010] [PMID: 29990861]
- Gence L, Fernezelian D, Bringart M, Veeren B, Christophe A, Brion F, et al. Hypericum lanceolatum Lam. medicinal plant: potential toxicity and therapeutic effects based on a zebrafish model. Front Pharmacol. 2022;13. [DOI: 10.3389/fphar.2022.832928] [PMID: 35359845]