Introduction
Orlistat is an anti-obesity medication derivative of the endogenous lipstatin found in Streptomyces toxytricini approved by the US Food and Drug Administration (FDA). The maximum benefit of orlistat is achieved when it is combined with a balanced diet and regular exercise [1]. The American Association of Pediatrics guidelines recommend orlistat for managing obesity in children aged 12 and older [2]. In a study involving 105 pediatric patients, orlistat exposures among young children were managed by decontamination and had favorable outcomes with few gastrointestinal adverse clinical effects [3]. Despite orlistat's promise as an anti-obesity therapy, its systemic effects necessitate careful consideration, particularly given that the orlistat/melatonin combination therapy demonstrates greater safety and effectiveness compared to monotherapy with either agent [4, 5].
Orlistat is a safe and effective drug for treating obesity by acting as an inhibitor of pancreatic and gastric lipases, reducing fat absorption. There is also concern that it may be associated with an increased risk of serious liver events [6]. Orlistat caused an apparent injury in the liver, kidney, and cerebellum of obese rats [7]. Also, apoptosis is considered a possible mechanism of testicular toxicity for orlistat in a dose of 120 mg/kg [8]. The demonstrated protective antioxidant effect of vitamin C against orlistat-induced damage in salivary glands supports the view that orlistat has a detrimental impact, further substantiated by its metabolic toxicity observed in D. magna [9, 10]. Approximately 95% to 97% of the orlistat is unabsorbed and excreted in feces [11]. Research indicates that orlistat was among the highest indicators of exposure in fish plasma [10].
Materials and Methods
Preparation of the high-fat diet
The high-fat food was prepared according to the method [12] an isoenergetic high-fat-induced diet consists of 30% calories from animal fat (30% fat, 50-52% CH, 18-20% protein 1-2% vitamins and minerals; 210 kcal), the diet was prepared, and necessary vitamins and minerals were added. For the fatty diet, the chow, in powder form, was mixed with 30% added melted animal abdominal fat until it became homogenous in a dough-like consistency. Obtained chow blocks were dried and used for feeding, and this food was given to the targeted groups only.
Orlistat drug
Orlistat drug obtained from a pharmacy in the city of Diwaniyah/ Iraq. Three doses was used to test for orlistat toxicity 360, 480 and 600 mg/kg of body weight. After the full daily dose of Orlistat drug was dissolved in distilled water, each animal was given 1 ml orally, using a special syringe for this purpose. These doses were used based on previous research [13, 14, 8].
Experimental animals and design
In this study, 25 animals of male Albino Rats were used, with an average weight of 190±5g, two months age. They were obtained from the Animal House, College of Science, Al-Qadisiyah University. Animals were acclimated for two weeks before the experiment. Experimental animals were placed in special plastic cages designed for this purpose at room temperature (22-25 °C) and in a photoperiod of 12h/day. The experimental period was 60 days. Animals were divided randomly into five equal groups (5 rats for each group) as follows: The control group in which healthy untreated rats was orally administered saline, the high-fat diet (HFD) group, and the HFD treated daily by oral gavage with Orlistat (OS) at doses of 360, 480, and 600 mg/kg for 60 days.
After 60 days had ended, the rats were anesthetized with isoflurane and sacrificed. Then they were desiccated, blood was collected from the heart Vena Cava, and collected in test tubes containing heparin as an anticoagulant. 1 mL was collected for hematological parameters. Plasma was separated from the blood by centrifuging at 860×g for 20 min and preserved at -80 °C for analysis. Heart and Lung were isolated, then washed with saline and kept at -80 ˚C for further biochemical studies. Parts of selected organs used for histological studies are kept in formalin (10%).
Hematological analysis
Hematology samples were analyzed with automated blood cell counter Sysmex XT-2000i and Olympus AU 400 analyzers right after drawing blood. The hematology parameters included Lymphocytes Percentage Test (LY), Neutrophils Percentage Test (NE), Eosinophilia (EO), Basophils Test (BA).
Biochemical parameters
Random Blood Sugar (RBS) levels [15], Total serum bilirubin (TSB) according to Price [16]. Serum globulin (GLO) concentration was determined indirectly by the method of Watson [17].
Markers of oxidative stress
The ROS level was determined according to Erel [18] the new automated colorimetric method is designed for biological fluids. The fundamental principle involves the oxidation of ferrous ions (Fe²⁺) to ferric ions (Fe³⁺) by various oxidant molecules present in the sample, all within an acidic environment. To increase the reaction rate and prevent protein precipitation, the assay incorporates glycerol to enhance the oxidation chain reaction and o-dianisidine to stabilize the ferrous ion reagent. The resultant ferric ions (Fe³⁺) then react with xylenol orange to produce a colored complex. The intensity of this final color is measured spectrophotometrically and is directly proportional to the total concentration of oxidants.
Peroxiredoxin level was estimated according to Mohsin [19] utilizing the spectrophotometric measurement of organic or inorganic peroxide. In the current protocol, Prx activity was measured by incubating the enzyme samples with 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (HEPES) buffer, containing suitable concentrations of the substrates 1,4-dithio-DL-threitol (DTT), and organic or inorganic peroxide. At the end of incubation, a sulfosalicylic acid (SSA)/ammonium ferrous sulfate solution was added to stop the enzymatic reaction. SSA acted as a legend to link ferric ions formed from the reaction between ferric ammonium sulfate (FAS) and residual peroxide, producing a maroon-colored ferrisulfosalicylate complex, spectrophotometrically measured at 500 nm. The current assay is free from interferences caused by different types of biomolecules in the studied sample. The method does not require strong, concentrated acids to stop the enzymatic reaction, does not require protein precipitation, and can be completed in a short period.
In addition, Glutathione -S-transferase it has been measured according to Habig [20] the reaction mixture consists of 1.575 ml sodium phosphate buffer (0.1 M, pH 7.4), 0.2 ml reduced glutathione (1 mM), 0.025 ml CDNB (1 mM), and 0.2 ml serum in a total volume of 2.0 ml. The changes in the absorbance were recorded at 340 nm, and enzyme activity was calculated using a molar extinction coefficient of 9.6×10 3 M −1 cm −1.
Histology studies
Parts of Heart and Lung tissues were fixed in 10 % formaldehyde solution, embedded in paraffin wax, and cut with microtome for 5µ thick sections. The sections were stained by Hematoxylin and Eosin (H&E) stains and microscopically studied to evaluate its morphology [21].
Statistical analysis
Data were fed to the computer and analyzed using the IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp) The Shapiro-Wilk test was used to verify the normality of distribution. Quantitative data were described using mean and standard error. The significance of the obtained results was judged at the 5% level. In addition, the test used is the F-test (ANOVA) for normally distributed quantitative variables, to compare more than two groups, and the Post Hoc test (Tukey) for pairwise comparisons.
Results
The results shown in Table 1 showed the effect of different doses of Orlistat on some hematological parameters. It was noted that the level of Lymphocytes Percentage (LY) did not change in all groups treated with either fats or Orlistat except for the five group (OS-600 mg/kg), where a decrease in the level of lymphocytes was noted. As for the level of Neutrophils Percentage (NE) it was noted that the level increased in the two groups treated with Orlistat 360 and 600 mg/kg when compared to the second group, and at the same time the levels of these two groups were equal or close to the first group, which is the control group, and the increase in Neutrophils may indicate an attempt to confront this inflammation caused by fats.
Table1. Effect of Varying Orlistat Doses on White Blood Cell Subsets in HFD-fed Male Rats
| Experimental groups |
Parameters |
| LY |
NE |
EO |
BA |
| Control |
90.07a ± 0.24 |
6.42a ± 0.38 |
1.51a ± 0.32 |
0.32a ± 0.09 |
| HFD |
94.68a ± 0.05 |
3.57b ± 0.01 |
0.38b ± 0.02 |
0.21a ± 0.02 |
| HFD+OS-360 |
92.47a ± 0.64 |
5.43a ± 0.65 |
0.79b ± 0.03 |
0.32a ± 0.05 |
| HFD+OS-480 |
93.68a ± 0.36 |
3.58b ± 0.31 |
0.54b ± 0.09 |
0.19a ± 0.04 |
| HFD+OS-600 |
60.57b ± 11.85 |
5.25ab ± 0.38 |
0.77b ± 0.03 |
0.12a ± 0.03 |
| F (p) |
7.440*(0.001*) |
9.715*(<0.001*) |
8.065*(<0.001*) |
2.821 (0.053) |
Results are expressed as Mean ± SD; SD: Standard Deviation, P-value: where a (P ≤ 0.05) was considered statistically significant, LY: Lymphocytes, NE: Neutrophils, EO: Eosinophils, BA: Basophil.
On the other hand, the analysis of Eosinophilia (EO) indicated a decrease in all groups treated with either fats or Orlistat (in all three doses) compared to the first group, and this may indicate the presence of common factors between fats and drug that affected the production or function of eosinophilia or they may have an inhibitory effect on some aspects of the immune response, leading to a decrease in the production of eosinophilia.
Basophils (BA) was less sensitive to changes in diet or drug compared to other types of white blood cells.
In Table 2 some biochemical parameters are shown and the results indicate that Orlistat is effective in reducing RBS and that this effect depends on the dose, as eating large amounts of fat can lead to insulin resistance and high blood sugar levels, which is what we noticed in the second group when compared to the first group, while Orlistat reduced the amount of calories absorbed by the body and helped improve blood sugar levels, and this was also noticed in the last three groups that were treated with Orlistat, as blood sugar levels gradually decreased until they reached their lowest level at a dose of 600 mg/kg.
The increase in bilirubin production (TSB) in the fat group may be due to increased red blood cell breakdown, and this increase was in the second group, while the increase in TSB in the groups treated with Orlistat is due to the effect of the drug on the absorption of fats and fat-soluble vitamins.
The level of GLO was not affected in all groups except the group treated with a dose of 480 mg/kg, as this dose may be able to affect some enzymes or hormones that regulate the work of GLO.
These results indicate that orlistat lowers RBS, but may raise TSB without affecting GLO, suggesting different effects of the drug on glucose, lipid, and bilirubin metabolism.
Table 2. Effect of Varying Orlistat Doses on RBS, Bilirubin, and Globulin in HFD-fed Male Rats.
| Experimental groups |
Parameters |
| RBS |
TSB |
GLO |
| Control |
177.0ab ± 8.42 |
0.60b ± 0.08 |
3.50b ± 0.04 |
| HFD |
188.0a ± 10.0 |
0.80ab ± 0.04 |
3.50b ± 0.12 |
HFD+OS-360
|
164.0abc ± 15.44 |
0.88a ± 0.05 |
3.50b ± 0.04 |
HFD+OS-480
|
147.0bc ± 2.28 |
0.87a ± 0.03 |
5.04a ± 0.56 |
HFD+OS-600
|
132.1c ± 2.18 |
0.80ab ± 0.04 |
3.54b ± 0.22 |
| F (p) |
6.040*(0.002*) |
5.400*(0.004*) |
6.225*(0.002*) |
Results are expressed as Mean ± SD; SD: Standard Deviation, P-value: where a (P ≤ 0.05) was considered statistically significant, RBS; Random Blood Sugar, TSB; Total Serum Bilirubin, GLO; Globulin.
In the Table 3 shows some oxidative stress in the heart tissues, the absence of significant changes in GST levels in all treatment groups indicates that high-fat diet and orlistat have no significant effect on GST levels.
As for ROS analysis, the fat group decreased compared to the control group, and this decrease may be due to antioxidant defense mechanisms stimulated by the high-fat diet. While an increase in ROS was observed in the orlistat groups, this may be due to increased production of free radicals due to the effect of the drug on cellular metabolic processes.
When conducting this analysis, we did not observe changes in the fat group compared to the control, which may be evidence that the high-fat diet does not have a significant effect on the levels of this enzyme, but it increased in the group treated with Orlistat at a dose of 360 mg/kg, which is a response to the oxidative stress that the drug may cause, while at a dose of 600 mg/kg, the enzyme level decreased compared to the fat, which is due to the exhaustion of the cells' ability to produce the enzyme with high doses of the drug, or due to an inhibitory effect of the drug on enzyme production. These results indicate that Orlistat may increase oxidative stress, as evidenced by the increase in ROS, and affect Peroxiredoxin in a dose-dependent manner, while it does not affect GST.
Table 3. Effect of Varying Orlistat Doses on Oxidative Stress Markers in heart HFD-fed Male Rats.
| Experimental groups |
Parameters |
| GST (U/mg protein) |
ROS (µ mol/L) |
Peroxiredoxin (U/mg protein) |
| Control |
1.48a ± 0.20 |
16.31ab ± 0.13 |
3.60ab ± 0.02 |
| HFD |
1.71a ± 0.37 |
15.51b ± 0.26 |
3.51ab ± 0.11 |
HFD+OS-360
|
1.48a ± 0.04 |
15.99ab ± 0.10 |
3.68a ± 0.01 |
HFD+OS-480
|
2.23a ± 0.07 |
16.51a ± 0.25 |
3.57ab ± 0.04 |
HFD+OS-600
|
1.42a ± 0.31 |
16.54a ± 0.16 |
3.38b ± 0.02 |
| F (p) |
2.010(0.132) |
5.156*(0.005*) |
4.371*(0.011*) |
Results are expressed as Mean ± SD; SD: Standard Deviation, P-value: where a (P ≤ 0.05) was considered statistically significant, GST; Glutathione S-Transferase, ROS; Reactive Oxygen Species, Peroxiredoxin.
Table 4 shows the levels of GST, ROS and Peroxiredoxin in lung tissue. The results showed no significant differences in GST levels in all groups except the group that received a dose of 360 mg/kg of Orlistat when compared to the second group.
As for the ROS analysis, it was found that there was a decrease in the group that received fats compared to the control, which indicates that the high-fat diet reduces ROS in lung tissues, as consuming large amounts of fats leads to increased production of free radicals, which stimulates the body to increase the production of antioxidants to deal with these radicals, as well as a decrease in the levels in the third and fourth groups, while at the dose of 600 mg/kg it increased compared to the second group, where it became almost equal to the control group, and this is because Orlistat affected the functions of mitochondria in lung cells, which may lead to increased production of free radicals.
Peroxiredoxin, The results showed an increase in the second group compared to the control, while it decreased in the third group compared to the group that received fats.
Histology is a sensitive tool not only for the detection of dose effects but also for providing insight into the onset, and mechanism (s) of action. Histology provides a rapid method to detect effects of pathogens in different organs and it can be considered as the indicator for abnormal condition for environment.
Table 4. Effect of Varying Orlistat Doses on Oxidative Stress Markers in lung HFD-fed Male Rats.
| Experimental groups |
Parameters |
| GST (U/mg protein) |
ROS (µ mol/L) |
Peroxiredoxin (U/mg protein) |
|
| Control |
1.94b ± 0.73 |
18.41a ± 0.98 |
3.53a ± 0.08 |
|
| HFD |
1.98b ± 0.30 |
15.75b ± 0.15 |
3.68a ± 0.01 |
|
HFD+OS-360
|
4.49a ± 0.99 |
15.66b ± 0.94 |
1.98b ± 0.61 |
|
HFD+OS-480
|
0.73b ± 0.12 |
16.67ab ± 0.01 |
3.69a ± 0.03 |
|
HFD+OS-600
|
1.09b ± 0.34 |
18.62a ± 0.31 |
3.48a ± 0.05 |
|
| F (p) |
6.188*(0.002*) |
5.159*(0.005*) |
6.967*(0.001*) |
|
Results are expressed as Mean ± SD; SD: Standard Deviation, P-value: where a (P ≤ 0.05) was considered statistically significant, GST; Glutathione S-Transferase, ROS; Reactive Oxygen Species, Peroxiredoxin.
Microscopic images of heart tissue in the first group (Control) showed that the tissue was normal with oval nuclei located in the center, and the myocardial fibers were of normal structure and branched (Figure 1). The second group (HFD) showed significant histological changes compared to the first group, including myocarditis, fibrosis, necrosis, possible formation of blood clots, and the presence of pigment deposits. These results indicate that the high-fat diet has resulted in significant damage to heart tissue (Figure 2).
The orlistat-treated groups also showed abnormal tissue changes, but to varying degrees. (HFD+ OS-360) showed edema, fibrosis, and necrosis. (HFD+ OS-480) showed hemorrhage, edema, and changes in tissue architecture. (HFD+ OS-600) showed hemorrhage, tissue architecture damage, and areas of low cell density (Figures 3, 4, and 5). These results suggest that orlistat, at different doses, may have deleterious effects on heart tissue and may not protect against high-fat diet-induced damage.
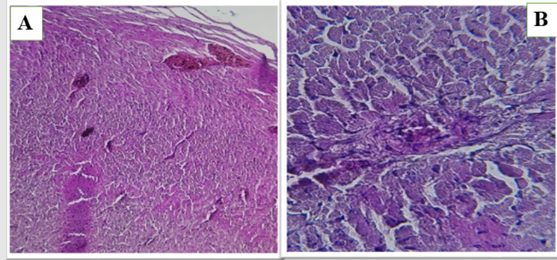
Figure 1 (A and B). Show the control group in heart of male rats.
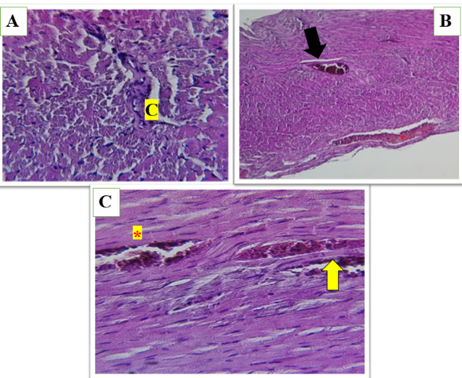
Figure 2 (A, B and C). Show the high-fat diet (HFD) group in heart of male rats, asterisks represent dilated congestion of blood vessel. yellow arrow represent the accumulation of mononuclear cells associated with inflammation. Black arrow represents perivascular fat surrounding the blood vessel. congestion between myocardial fibers (C) loss of normal architecture and widespread fragmentation and degeneration of muscle fibers.
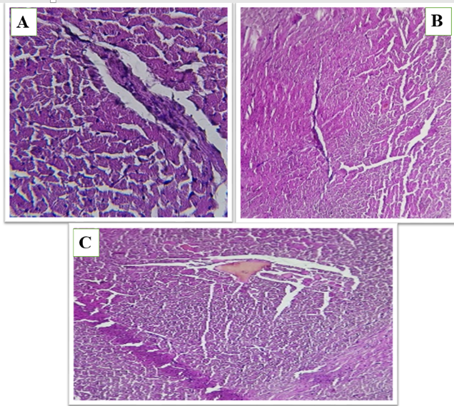
Figure 3 (A, B and C). Show the fed a high-fat diet (HFD) and treated with Orlistat (OS-360) in heart of male rats, Congested vessels, perivascular fat, and degenerated myocardial fibers with architectural loss.
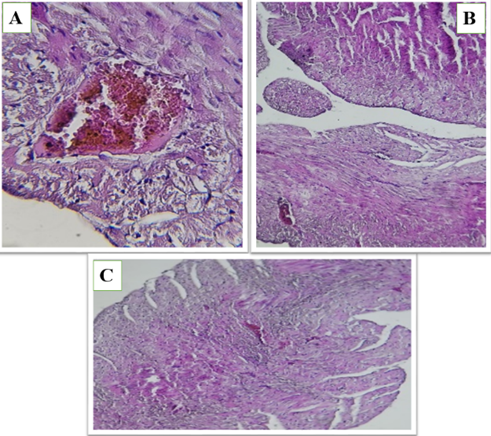
Figure 4 (A, B and C). Show the fed a high-fat diet (HFD) and treated with Orlistat (OS-480) in heart of male rats, hemorrhage, edema, and alterations in tissue structure, indicating potential cardiac damage. The tissue exhibits varying degrees of cellular disorganization and structural abnormalities.
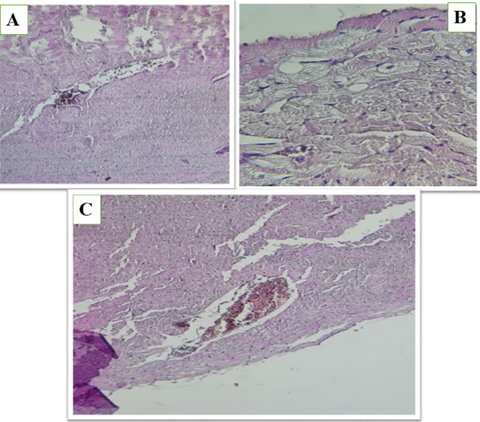
Figure 5 (A, B and C). Show the fed a high-fat diet (HFD) and treated with Orlistat (OS-600) in heart of male rats, hemorrhage, structural disruption, and areas of reduced cellular density, suggesting potential adverse effects on the cardiac tissue. The overall appearance indicates a compromised tissue integrity.
The heart tissue images across all groups revealed a spectrum of pathological changes, ranging from normal tissue in the control group to significant damage in the high-fat diet and orlistat-treated groups.
Observed alterations included inflammation, fibrosis, necrosis, hemorrhage, and edema, indicating varying degrees of cardiac tissue compromise. The results indicates that high fat diet has negative effects on the heart tissue. , and that Orlistat drug with different doses, has also negative effects on the heart tissues.
Microscopic images of lungs tissue in the first group (Control) show normal lung tissue architecture with intact alveolar spaces and bronchioles. No significant pathological abnormalities are observed, indicating healthy lung tissue in the control group (Figure 6).
The second group (HFD) exhibited significant histological changes compared to the first group, including inflammatory cell infiltration and alterations in alveolar spaces.These findings suggest that a high-fat diet induced pathological changes in the lung tissue (Figure 7).
The orlistat-treated groups also showed structural changes in the lung tissue, but to varying degrees. All treated groups (3, 4, 5) shows noticeable changes in alveolar spaces and bronchiolar structure (Figures 8, 9, and 10). These results indicate that orlistat, at different doses, may have adverse effects on lung tissue, and may not protect against damage caused by a high-fat diet.
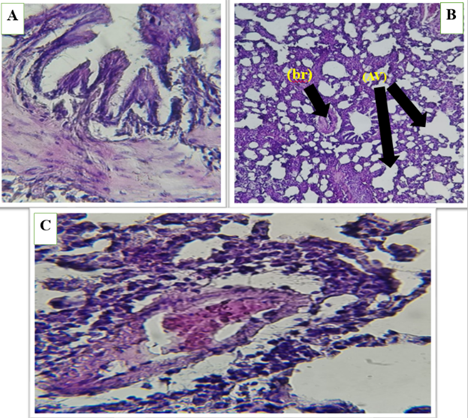
Figure 6 (A, B and C) . Show the control group in lung of male rats, normal bronchioles (br), lung with alveolar sacs (AV) , expanded and non-filled alveoli , thin septa , and blood vessels.
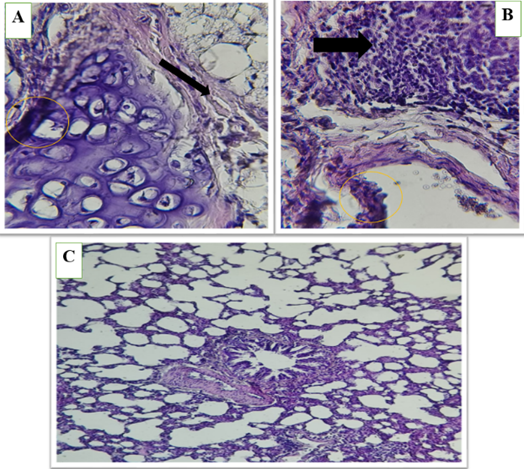
Figure 7 (A, B and C). Show the high-fat diet (HFD) group in lung of male rats, alveolar septum (*) thickening and slight leukocyte infiltration (blavk arrows), Congestion of blood vessels ( ). Acute bronchiolitis (circle) surrounded by acute pneumonia.
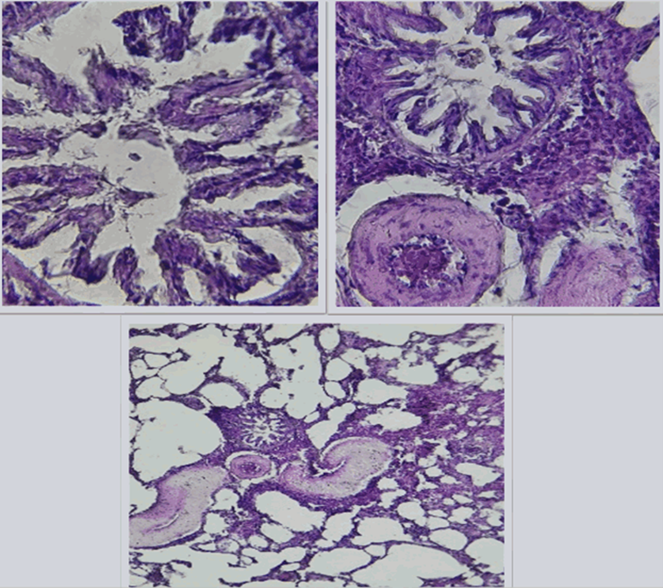
Figure 8 (A, B and C). Show the fed a high-fat diet (HFD) and treated with Orlistat (OS-360) in lung of male rats, noticeable structural changes, including altered bronchiolar architecture and increased cellularity. These findings suggest potential pathological changes compared to the control group, indicating possible lung tissue compromisee.
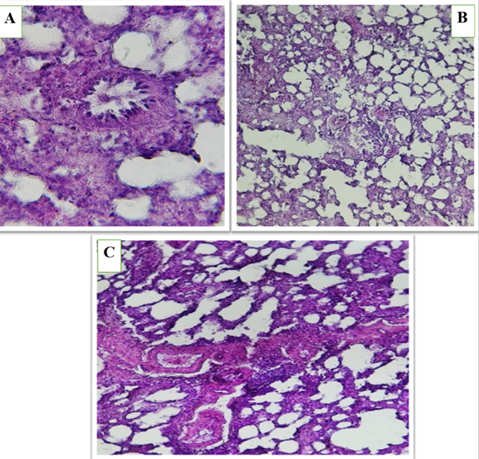
Figure 9 (A, B and C). Show the fed a high-fat diet (HFD) and treated with Orlistat (OS-480) in lung of male rats, Alveolar septum thickening and mild leukocyte infiltration.
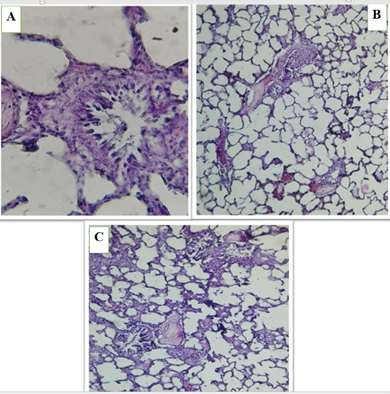
Figure 10 (A, B and C). Show the fed a high-fat diet (HFD) and treated with Orlistat (OS-600) in lung of male rats, Sever leukocyte infiltration, thickening alveolar septum and congestion of blood vessels..
Discussion
Changes in these tests suggest that a high-fat diet and orlistat, especially at high doses, affect the immune system through different mechanisms, possibly including affecting the bone marrow, inducing an inflammatory response, and affecting the functions of some types of white blood cells. Hasan et al in 2024 they discussed some blood parameters and the effect of Orlistat on them. They indicated that Orlistat affects white blood cells, indicating its effect on the immune system and there are changes in the proportions of lymphocytes and neutrophils [22].
In the same context, Hamza and Alsolami explained that orlistat administration in both protective and therapeutic groups showed reduction in the blood glucose level [23]. Also, Shalaby was observed that Orlistat was reduce the concentration of bilirubin [24]. And Aljamal et al in 2023 showed that orlistat at a dose of 4 mg/kg body weight reduced the concentration of globulin in mice with hypercholesterolemia [25].
Peroxiredoxin is an antioxidant enzyme that protects cells from damage caused by free radicals. Othman et al., 2022 used a dose of 10 mg/kg/day of orlistat, which was shown to improve antioxidant levels and increase their activity [26]. It also reduced oxidative stress factors. In another study for, also used a dose of 10 mg/kg/day of orlistat, Orlistat increased the activity of antioxidant enzymes in heart tissues. Alsunbul et al., 2024 was uses "Orli-Free" and "Orli-Nanocrystals" It increased the activity of antioxidant enzymes, increased the expression of Nrf2 and HO-1, which are antioxidant factors in heart tissue [27].
In a study on the pancreas organ conducted by El-Sayed et al., 2025, they concluded that in the group that received orlistat at a dose of 30 mg/kg, there were gaps in the pancreatic cells, while in the group that received a dose of 40 mg/kg, there was destruction of the pancreatic cells with the presence of fat cells and infiltration of inflammatory cells [28]. In addition, dilatation of the pancreatic ducts with retention of secretions was observed in both groups. These results indicate that orlistat can cause inflammation and fatty deterioration of the pancreas, and that these effects are dose-dependent. Also, Elbakary and Bayomy, 2011 advised taking Orlistat under medical supervision after using it at a dose of 32 mg/kg and concluding that it had harmful effects on the pancreatic vesicles [29].
Laag et al., 2024 [30] noted that orlistat may help reduce testicular damage caused by obesity, but its effect may vary depending on the presence of a high-fat diet and the dose, where the doses that uses in this study 30 mg/kg for 30 day. In addition, gave the rats three different doses of orlistat (50, 100 and 150 mg/kg/day) and the histological study showed pathological changes in the testicles. These pathological changes increased with increasing concentration of orlistat. They found that the concentration of 150 mg/kg/day was the most effective in showing these pathological changes [31].
Conducted a study on three organs with different doses 10, 30, 40, 50, 100 and 150 mg/kg/day , the study showed tissue changes in the heart that it improves changes in the left ventricular muscle, restores the elastic tissue component, and reverses the increased deposition of collagen in obese male mice caused by a high-fat diet, while in the testicles, orlistat causes pathological changes in the testicles, which increase with increasing concentration of orlistat, and in the pancreas, it leads to inflammation, fatty deterioration, and weakness in insulin production in the pancreas, and these effects depend on the dose [32].
Conclusions
O The administration of Orlistat in obese rats induced significant dose-dependent oxidative stress and adverse effects in both cardiac and pulmonary tissues. Specifically, Orlistat increased Reactive Oxygen Species (ROS) and altered peroxiredoxin in the heart, and caused changes in Glutathione S-Transferase (GST) and ROS in the lung. Histological findings corroborated these results, showing damage (inflammation, fibrosis) in the heart and lung tissues across the treatment groups. These data suggest that while Orlistat effectively reduces Random Blood Sugar RBS, it may not protect against high-fat diet-induced cardiopulmonary damage and poses a risk of dose-dependent toxicity.
Data Access and Responsibility
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request “Upon request”.
Ethical Considerations
T The research was achieved according to the Guide for the Care and Use of Laboratory Animals (International Council for Laboratory Animal Science, ICLAS) and was approved by the local ethical guidelines of the Institutional Animal Care & Use Committee (IACUC), University of Al-Qadisiyah, Iraq (Aprroval No. 13) and all the methods were performed according to the guidelines and regulations of the same Committee.
Authors' Contributions
All authors contributed equally to the conception and design of the research, execution of the practical work, data analysis, drafting of the original manuscript, and critical revision of the final version.
Acknowledgement
The authors thank all those who contributed to the completion of this article.
Conflict of Interests
The authors declare no competing interests.
Consent to Publish
All authors have reviewed and approved the final version of the manuscript and consented to its publication.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Bansal AB, Patel P, Al Khalili Y. Orlistat. StatPearls Publishing. 2024. [LINK]
- Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151(2):e2022060640. [DOI:10.1542/peds.2022-060640] [PMID: 36622115]
- Forrester MB. Pattern of orlistat exposures in children aged 5 years or less. J emerg med. 2009;37(4):396-9. [DOI:10.1016/j.jemermed.2007.10.052] [PMID: 18403165]
- Yousef MI, Ibadi EA, Kamel MA, El-Banna S. Curtailment of nephrotoxicity of polyethylene glycol via moringa oleifera leaf extract in male rats. J Pharm Neg Result. 2022;13(3):1003-10. [DOI: 10.47750/pnr.2022.13.03.157]
- Ibadi EA, Yousef MI, Kamel MA, El-Banna S. Hepatotoxicity of polyethylene glycol and possible protection using moringa oleifera leaves extract (MOLE). J Med Chem Sci. 2023;6:907-19. [DOI:10.26655/JMCHEMSCI.2023.4.23]
- Youssef S. Light and electron microscopic study of the effect of orlistat on the liver of adult male albino rats and the possible protective role of β-carotene. Forensic Med Anatomy Rese. 2018;6(2):20-36. [DOI: 10.4236/fmar.2018.62003]
- Arisha SM, Kandil EH. Hepatorenal and cerebellar anti-toxic effects of curcumin against orlistat associated toxicity in obese male albino rats. IJCBR. 2022;6(4):13-30. [DOI:10.21608/JCBR.2022.168085.1280]
- Sabik L, Metwally DE, Mohamed E, Elfakharany Y. Toxic effects of orlistat and green tea extract on testes of adult albino rats:(comparative study). Zagazig J Forensic Med Toxicol. 2022;20(1):99-120. [DOI: 10.21608/zjfm.2021.96396.1092]
- Guo J, Sinclair CJ, Selby K, Boxall AB. Toxicological and ecotoxicological risk‐based prioritization of pharmaceuticals in the natural environment. Environ Toxicol Chem. 2016;35(6):1550-9. [DOI: 10.1002/etc.3319] [PMID: 26799673]
- Aubakirova B, Beisenova R, Boxall AB. Prioritization of pharmaceuticals based on risks to aquatic environments in Kazakhstan. Integr Environ Assess Manag. 2017;13(5):832-9. [DOI: 10.1002/ieam.1895] [PMID: 28120523]
- McClendon KS, Riche DM, Uwaifo GI. Orlistat: current status in clinical therapeutics. Expert Opin Drug Saf. 2009;8(6):727-44. [DOI: 10.1517/14740330903321485] [PMID: 19998527]
- Altunkaynak Z. Effects of high fat diet induced obesity on female rat livers (a histochemical study). Eur J Gen Med. 2005;2(3):100-9. [DOI: 10.29333/ejgm/82319]
- Harp JB. An assessment of the efficacy and safety of orlistat for the long-term management of obesity. J Nutr Biochem. 1998;9(9):516-21. [DOI: 10.1016/S0955-2863(98)00006-0]
- Abdulshaheed HG. Comparative physiology study of side effect between Xenical and Lipo-6 supplements which treated obese rabbets. Al-Qadisiyah J Pure Sci. 2022;27(1):1-9. [DOI: 10.29350/jops. 2022.27. 1.1542]
- Thompson RH. Colorimetric glucose oxidase method for blood glucose. Clin Chim Acta. 1966;13(1):133-5. [DOI: 10.1016/0009-8981(66)90281-6] [PMID: 5917676]
- Price CP, Bossuyt PM, Bruns DE. Tietz fundamentals of clinical chemistry. Medicine. 1976;3(1).
- Watson D. Albumin and “total globulin” fractions of blood. Adv Clin Chem. 1966;8:237-303. [PMID: 5321719]
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103-11. [DOI:10.1016/j.clinbiochem.2005.08.008] [PMID: 16214125]
- Mohsin NY, Demir H, Hadwan MH, Hadwan AM, Mohammed RM. A new fluorescent method for measuring peroxiredoxin enzyme activity using monobromobimane. J Fluoresc. 2025;35(8):6357-65. [DOI:10.1007/s10895-024-03991-4] [PMID: 39441257]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130-9. [PMID: 4436300]
- Drury RA, Wallington EA. Carleton’s histological technique 5th ed. New York: Churchill Livingstone. 1980:270. [LINK]
- Hasan AF, Alankooshi AA, Modher MN, El-Naggar SA, El-Wahsh HM, El-Bagoury AE, et al. Artemisia annua extract ameliorates hepato-renal dysfunctions in obese rats. Opera Medica et Physiol. 2024;11(2):47-65. [DOI:10.24412/2500-2295-2024-2-47-65]
- Hamza RZ, Alsolami K. Ameliorative effects of Orlistat and metformin either alone or in combination on liver functions, structure, immunoreactivity and antioxidant enzymes in experimentally induced obesity in male rats. Heliyon. 2023;9(8):e18724. [DOI:10.1016/j.heliyon.2023.e18724] [PMID: 37600390]
- Shalaby HM, Tawfek NS, Abo-El Hussein BK, El-Ghany MS. The assessment of some biochemical and immunological effects by amphetamine and orlistat on obesity in rats. Food Public Health. 2014;4(4):185-92. [LINK]
- Aljamal A, Al Shawabkeh M, Amawi K, Alqadi T, Khwaldeh A. Physiological effects of fig leaf extract and orlistat on obesity, kidney and liver of rats. Pak J Biol Sci. 2023;26(9):458-62. [DOI:10.3923/pjbs.2023.458.462] [PMID: 38044695]
- Othman ZA, Zakaria Z, Suleiman JB, Mustaffa KM, Jalil NAC, Wan Ghazali WS, et al. Orlistat mitigates oxidative stress-linked myocardial damage via NF-κβ-and caspase-dependent activities in obese rats. Int J Mol Sci. 2022;23(18):10266. [DOI:10.3390/ijms231810266] [PMID:36142178]
- Alsunbul M, El-Masry TA, El Zahaby EI, Gaballa MMS, El-Nagar MMF. Potential protective effect of orlistat: a formulation of nanocrystals targeting inflammation, oxidative stress, and apoptosis in an experimental model of doxorubicin-induced cardiotoxicity. Pharmaceutics. 2024;16(11):1356. [DOI: 10.3390/pharmaceutics16111356] [PMID: 39598480]
- El-Sayed SM, El-Sayed GA, Mansour MA, Haridy Ahmed E, Kamar SA. A comparative study on the effect of melatonin and orlistat combination versus orlistat alone on high fat diet-induced hepatic changes in the adult male albino rats (a histological and morphometric study). Ultrastruct Pathol. 2025;49(1):20-38. [DOI: 10.1080/01913123.2024.2438380] [PMID: 39679624]
- Elbakary RH, Bayomy NA. Histological and immunohistochemical study of the effect of orlistat on the exocrine pancreas of adult female albino rat. Egypt J Histol. 2011;34(2):302-10. [DOI:10.1097/01.EHX.0000396877.23400.14]
- Laag SM, Mostafa MS, El-Sawaf ME, Abd Ellatif RA. Effect of orlistat versus lipo-6 black on the testis of adult obese albino rats: histological and histomorphometric study. Tanta Med J. 2024;52(2):142-50. [DOI: 10.4103/tmj.tmj_88_23]
- Sarhan DK, Abood AH. Histological effect of orlistat on the testes in the male rats rattus rattus. Int J Adv Multidisc Res Stud. 2023;3(3):563-8. [LINK]
- Anderson EL, Stephen SO, Eniola EM, Aderibigbe KO, Samson IM, Ruth OO, et al. Histological study on the effects of orlistat on left ventricular myocardium of high fat diet-fed adult male Wistar rats. Int J Veterin Sci. 2022;11(4):544-7. [DOI:10.47278/journal.ijvs/2021.140].










































 , Hussein Kamil Awad Awad2
, Hussein Kamil Awad Awad2 

 , Zainab Hudhi Farhoord Farhoord3
, Zainab Hudhi Farhoord Farhoord3 

 , Bilal Husam Jasim Jasim4
, Bilal Husam Jasim Jasim4 

 , Sabrean Farhan Jawad Jawad5
, Sabrean Farhan Jawad Jawad5 












